Question: Question 13 (2 points) Given the equation CO) + 3H2(0) - CHce) + H206) and 157.8 kJ of energy is released overall reaction will be
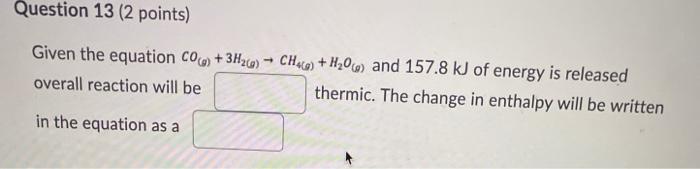
Question 13 (2 points) Given the equation CO) + 3H2(0) - CHce) + H206) and 157.8 kJ of energy is released overall reaction will be thermic. The change in enthalpy will be written in the equation as a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


