Question: Question 15 2 pts Calculation Problem #1: To complete this problem, work through all of the calculations and answer all parts of this question, and
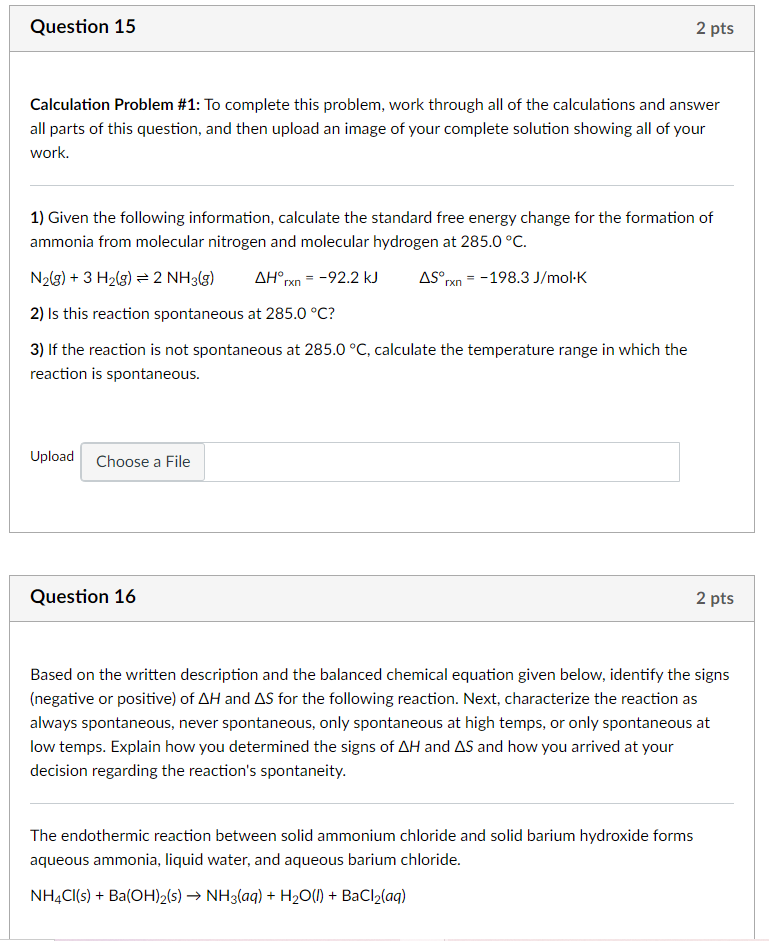
Question 15 2 pts Calculation Problem #1: To complete this problem, work through all of the calculations and answer all parts of this question, and then upload an image of your complete solution showing all of your work. 1) Given the following information, calculate the standard free energy change for the formation of ammonia from molecular nitrogen and molecular hydrogen at 285.0C. N2(g) + 3 H2(g) = 2 NH3(g) AHrx = -92.2 kJ -198.3 J/mol K 2) Is this reaction spontaneous at 285.0C? AS, rxn 3) If the reaction is not spontaneous at 285.0C, calculate the temperature range in which the reaction is spontaneous. Upload Choose a File Question 16 2 pts Based on the written description and the balanced chemical equation given below, identify the signs (negative or positive) of AH and AS for the following reaction. Next, characterize the reaction as always spontaneous, never spontaneous, only spontaneous at high temps, or only spontaneous at low temps. Explain how you determined the signs of AH and AS and how you arrived at your decision regarding the reaction's spontaneity. The endothermic reaction between solid ammonium chloride and solid barium hydroxide forms aqueous ammonia, liquid water, and aqueous barium chloride. NH4Cl(s) + Ba(OH)2(s) NH3(aq) + H2O(l) + BaCl2(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


