Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #11 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #11!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
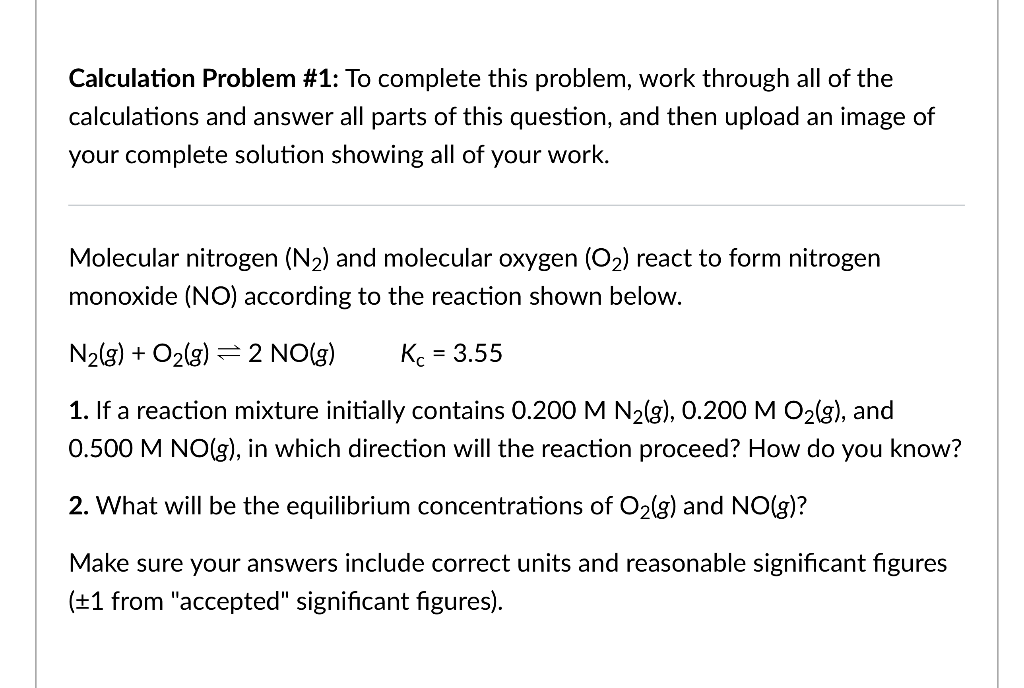
Calculation Problem \#1: To complete this problem, work through all of the calculations and answer all parts of this question, and then upload an image of your complete solution showing all of your work. Molecular nitrogen (N2) and molecular oxygen (O2) react to form nitrogen monoxide (NO) according to the reaction shown below. N2(g)+O2(g)2NO(g)Kc=3.55 1. If a reaction mixture initially contains 0.200MN2(g),0.200MO2(g), and 0.500MNO(g), in which direction will the reaction proceed? How do you know? 2. What will be the equilibrium concentrations of O2(g) and NO(g) ? Make sure your answers include correct units and reasonable significant figures ( 1 from "accepted" significant figures)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


