Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #7 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #7!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
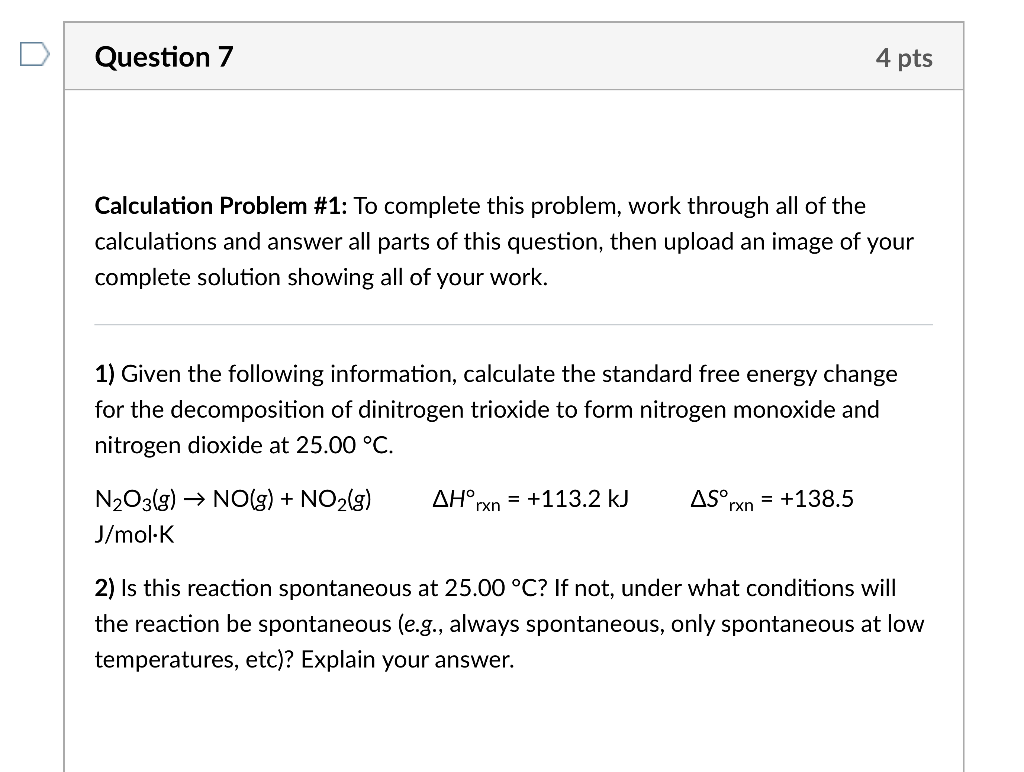
Calculation Problem \#1: To complete this problem, work through all of the calculations and answer all parts of this question, then upload an image of your complete solution showing all of your work. 1) Given the following information, calculate the standard free energy change for the decomposition of dinitrogen trioxide to form nitrogen monoxide and nitrogen dioxide at 25.00C. N2O3(g)NO(g)+NO2(g)Hrxn=+113.2kJSrxn=+138.5 J/molK 2) Is this reaction spontaneous at 25.00C ? If not, under what conditions will the reaction be spontaneous (e.g., always spontaneous, only spontaneous at low temperatures, etc)? Explain your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


