Question: Question 2 ( 2 . 0 points ) : One mole of a diatomic gas ( A ) are isothermally & reversibly compressed from 0
Question points:
One mole of a diatomic gas A are isothermally & reversibly compressed from bar and K to bar. If is a real gas which EOS is: calculate enthalpy change of the process using the following relation:
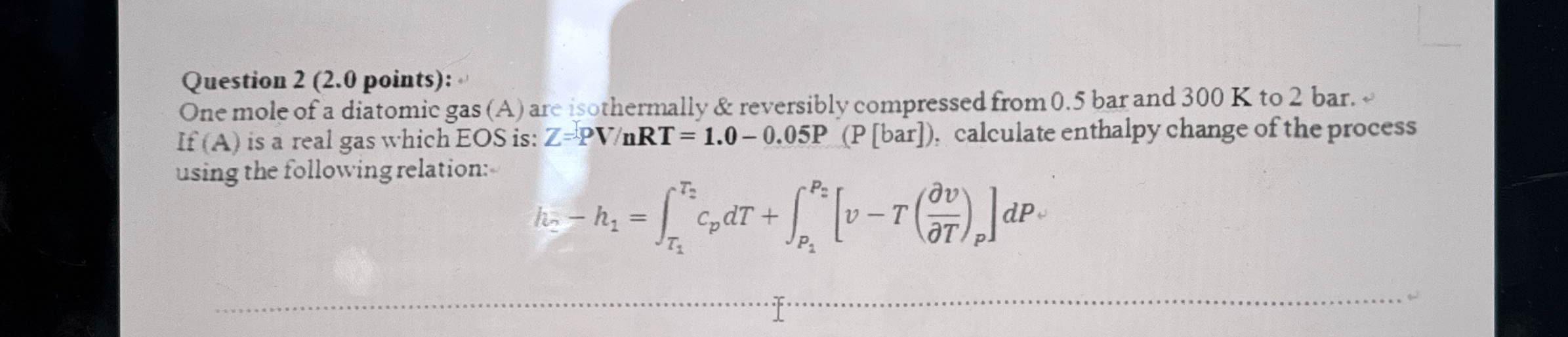
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


