Question: 2.1 The data in the table below was collected by sampling the contents of a batch reactor with time, where A is reacting. Assume
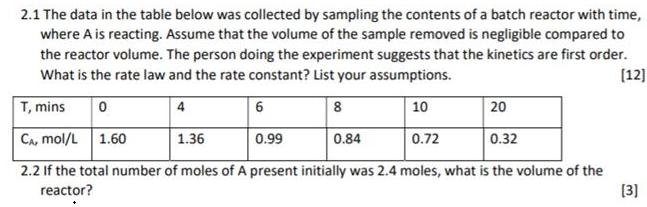
2.1 The data in the table below was collected by sampling the contents of a batch reactor with time, where A is reacting. Assume that the volume of the sample removed is negligible compared to the reactor volume. The person doing the experiment suggests that the kinetics are first order. What is the rate law and the rate constant? List your assumptions. (12] T, mins 4 6 8 10 20 CA, mol/L 1.60 1.36 0.99 0.84 0.72 0.32 2.2 If the total number of moles of A present initially was 2.4 moles, what is the volume of the [3] reactor?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
63624c2886bc1_236482.pdf
180 KBs PDF File
63624c2886bc1_236482.docx
120 KBs Word File


