Question: Question 2 (32pts) In lectures (Module 1.3), we derived a formula that relates the concentration of amphiphile molecules in aqueous solution (X1) to the concentration
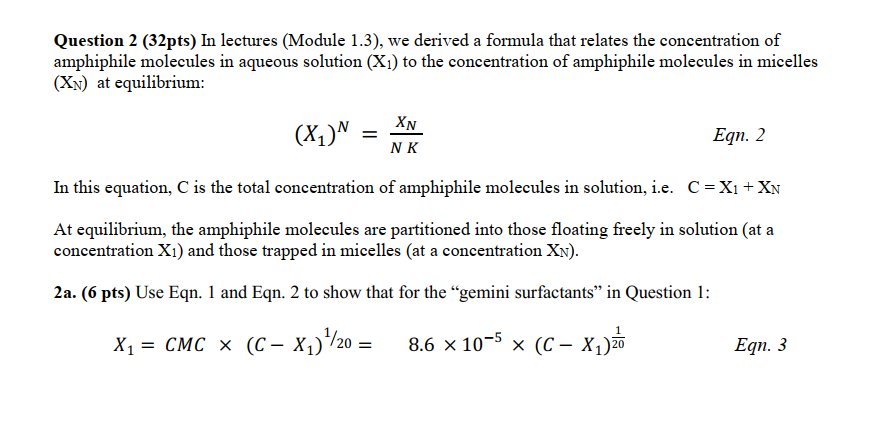
Question 2 (32pts) In lectures (Module 1.3), we derived a formula that relates the concentration of amphiphile molecules in aqueous solution (X1) to the concentration of amphiphile molecules in micelles (XN) at equilibrium: (X1)N=NKXN Eqn. 2 In this equation, C is the total concentration of amphiphile molecules in solution, i.e. C=X1+XN At equilibrium, the amphiphile molecules are partitioned into those floating freely in solution (at a concentration X1 ) and those trapped in micelles (at a concentration XN ). 2a. (6 pts) Use Eqn. 1 and Eqn. 2 to show that for the "gemini surfactants" in Question 1: X1=CMC(CX1)1/20=8.6105(CX1)201Eqn.3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


