Question: Question 2 (4 marks) A water solution (at 25C ) has a pH of 7.7 , and it contains 40mg/L calcium and 12mg magnesium. When
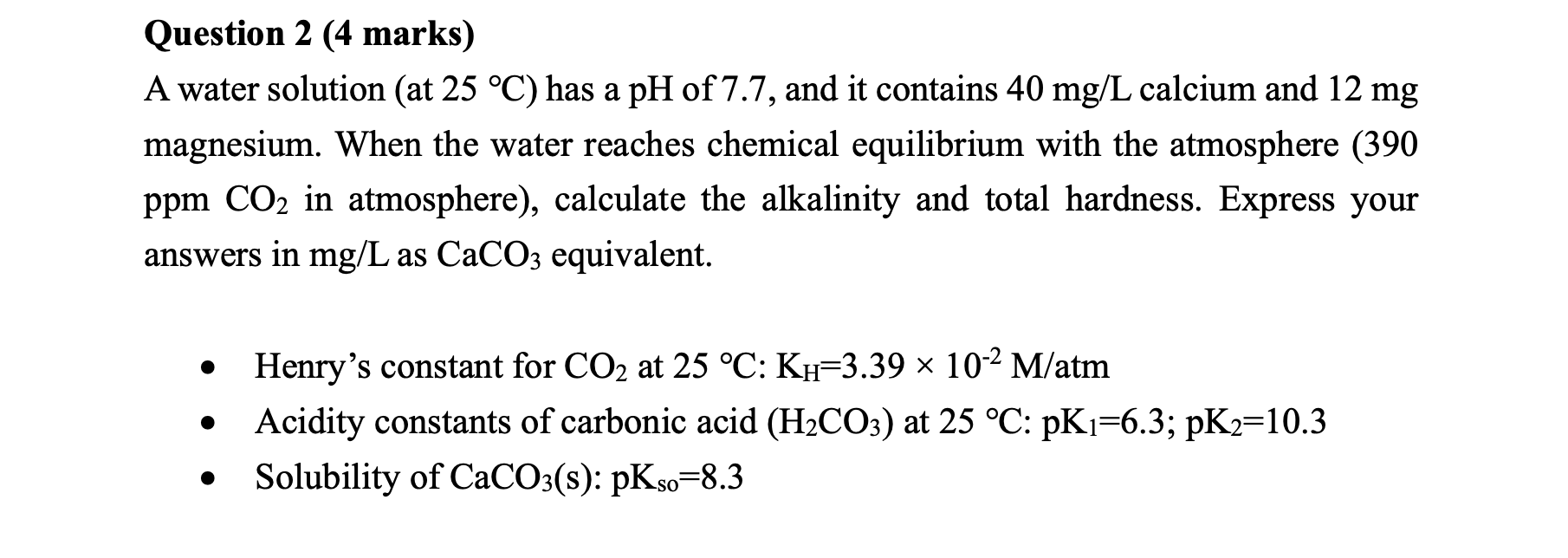
Question 2 (4 marks) A water solution (at 25C ) has a pH of 7.7 , and it contains 40mg/L calcium and 12mg magnesium. When the water reaches chemical equilibrium with the atmosphere (390 ppm CO2 in atmosphere), calculate the alkalinity and total hardness. Express your answers in mg/L as CaCO3 equivalent. - Henry's constant for CO2 at 25C:KH=3.39102M/atm - Acidity constants of carbonic acid (H2CO3) at 25C:pK1=6.3;pK2=10.3 - Solubility of CaCO3(s):pKso=8.3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


