Question: Question 2 5 pts In the double replacement reaction NaCl(aq) + Pb(NO3)2(aq) --> one of the products is PbCl2. What is the correct formula for
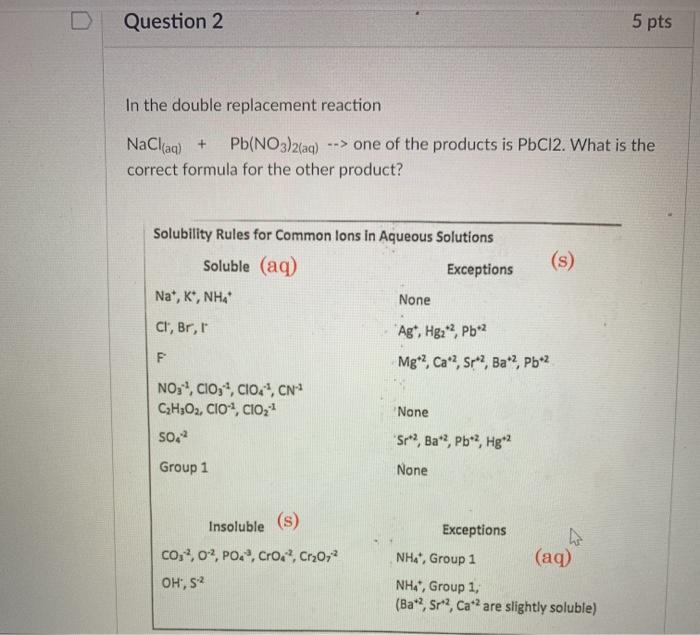
Question 2 5 pts In the double replacement reaction NaCl(aq) + Pb(NO3)2(aq) --> one of the products is PbCl2. What is the correct formula for the other product? Solubility Rules for Common lons in Aqueous Solutions Soluble (aq) Exceptions Nat, K', NHA None (s) Cl, Br, I Ag*, Hg2+2, Pb2 Mg2, Ca?, Sr*?, Ba?, Pb*2 NO:, CIO,, Clod, CN CzH;O2, CIO, CIO, SO None 'Sr"?, Ba, Pb*?, Hg"? Group 1 None Insoluble (s) c0, 3, O2, PO., Croa, Cr20,2 OH, 52 Exceptions NH, Group 1 (aq) NH, Group 1, (Ba 2, Sr, Ca*? are slightly soluble)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


