Question: question 2 and 3 plz clear steps Question 2 The data tabulated below were obtained for the decomposition reaction of a chemical compound at 50
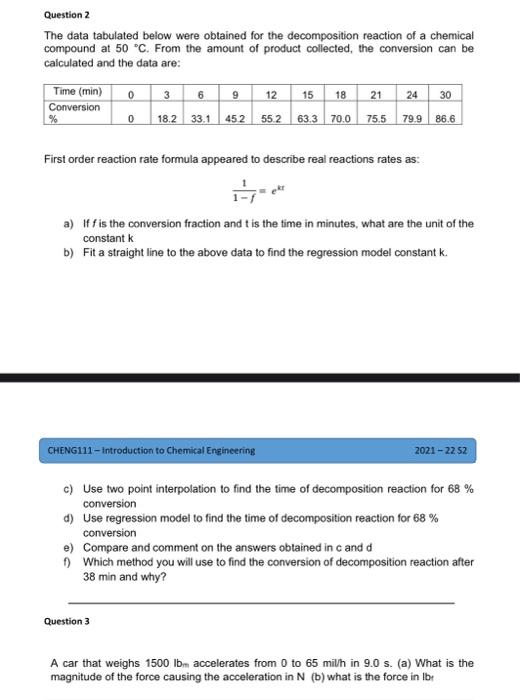
Question 2 The data tabulated below were obtained for the decomposition reaction of a chemical compound at 50 C. From the amount of product collected, the conversion can be calculated and the data are: Time (min) 3 15 18 21 24 Conversion 18.2 33.1 452 55.2 63.3 70.0 75.5 0 6 9 12 30 0 79.986.6 First order reaction rate formula appeared to describe real reactions rates as a) Iff is the conversion fraction and t is the time in minutes, what are the unit of the constant k b) Fit a straight line to the above data to find the regression model constant k. CHENG111 - Introduction to Chemical Engineering 2021 - 22 S2 C) Use two point interpolation to find the time of decomposition reaction for 68 % conversion d) Use regression model to find the time of decomposition reaction for 68 % conversion e) Compare and comment on the answers obtained in cand d 1) Which method you will use to find the conversion of decomposition reaction after 38 min and why? Question 3 A car that weighs 1500 lbn accelerates from 0 to 65 mil/h in 9.0 s. (a) What is the magnitude of the force causing the acceleration in N (b) what is the force in lb
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


