Question: Question 2 . Complex Reactions The 1 0 0 0 - liter isothermal CSTR is used to carry out the following reactions: A + B
Question Complex Reactions
The liter isothermal CSTR is used to carry out the following reactions:
The initial concentrations in the reactor are:
The volumetric feed rate is liters
If the corresponding digit in your student number is use liters
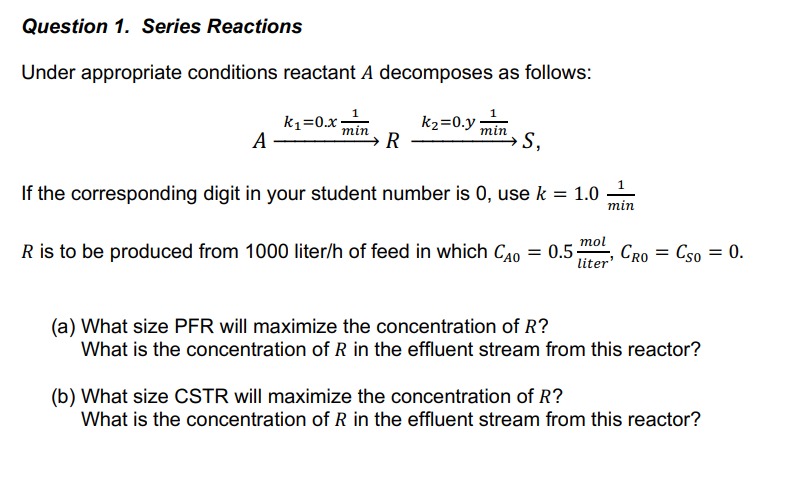
What is the ratio at the reactor exit?Question Series Reactions
Under appropriate conditions reactant A decomposes as follows:
If the corresponding digit in your student number is use
is to be produced from liter of feed in which
a What size PFR will maximize the concentration of
What is the concentration of in the effluent stream from this reactor?
b What size CSTR will maximize the concentration of
What is the concentration of in the effluent stream from this reactor?
X
Y
Z
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


