Question: Question 2 Ethane ( C 2 H 6 ) is burned with 5 0 % excess air. The percentage conversion of ethane is 9 0
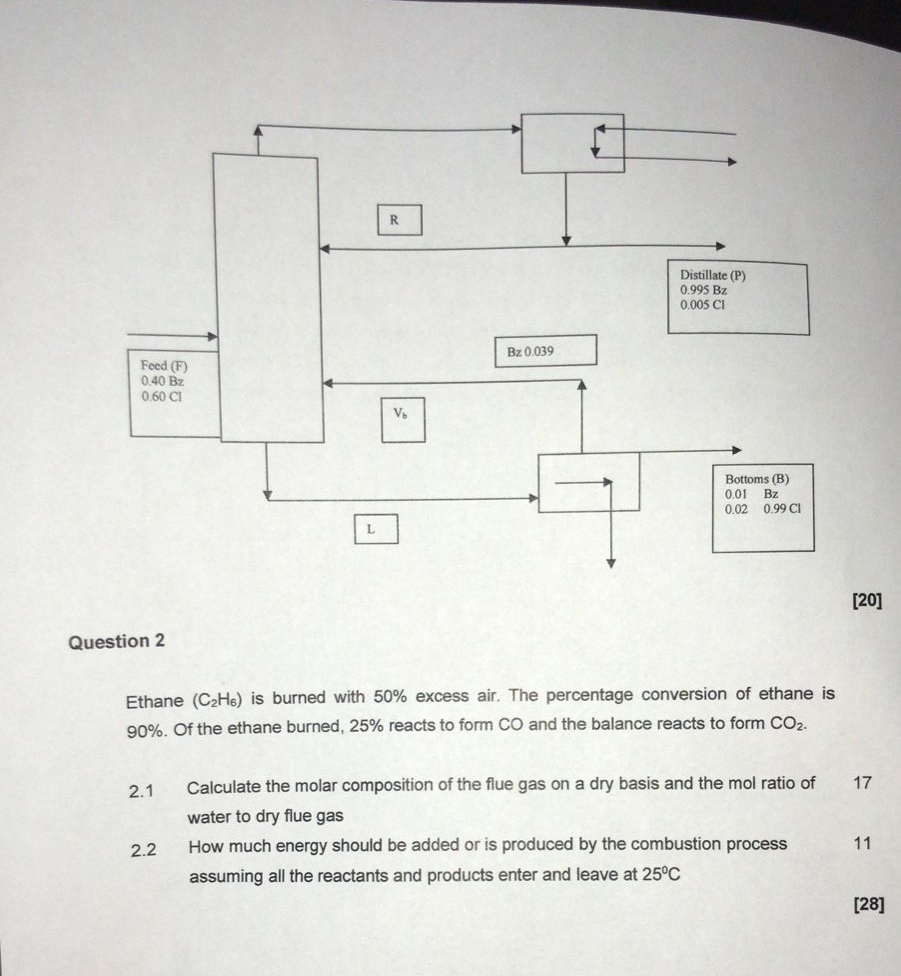
Question
Ethane is burned with excess air. The percentage conversion of ethane is Of the ethane burned, reacts to form and the balance reacts to form
Calculate the molar composition of the flue gas on a dry basis and the mol ratio of
water to dry flue gas
How much energy should be added or is produced by the combustion process
assuming all the reactants and products enter and leave at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


