Question: Question 3 (55 points) A mixture containing 50.0 wt. % Ethanol (E) and 50.0 wt. % water (W) is to be separated into two streams
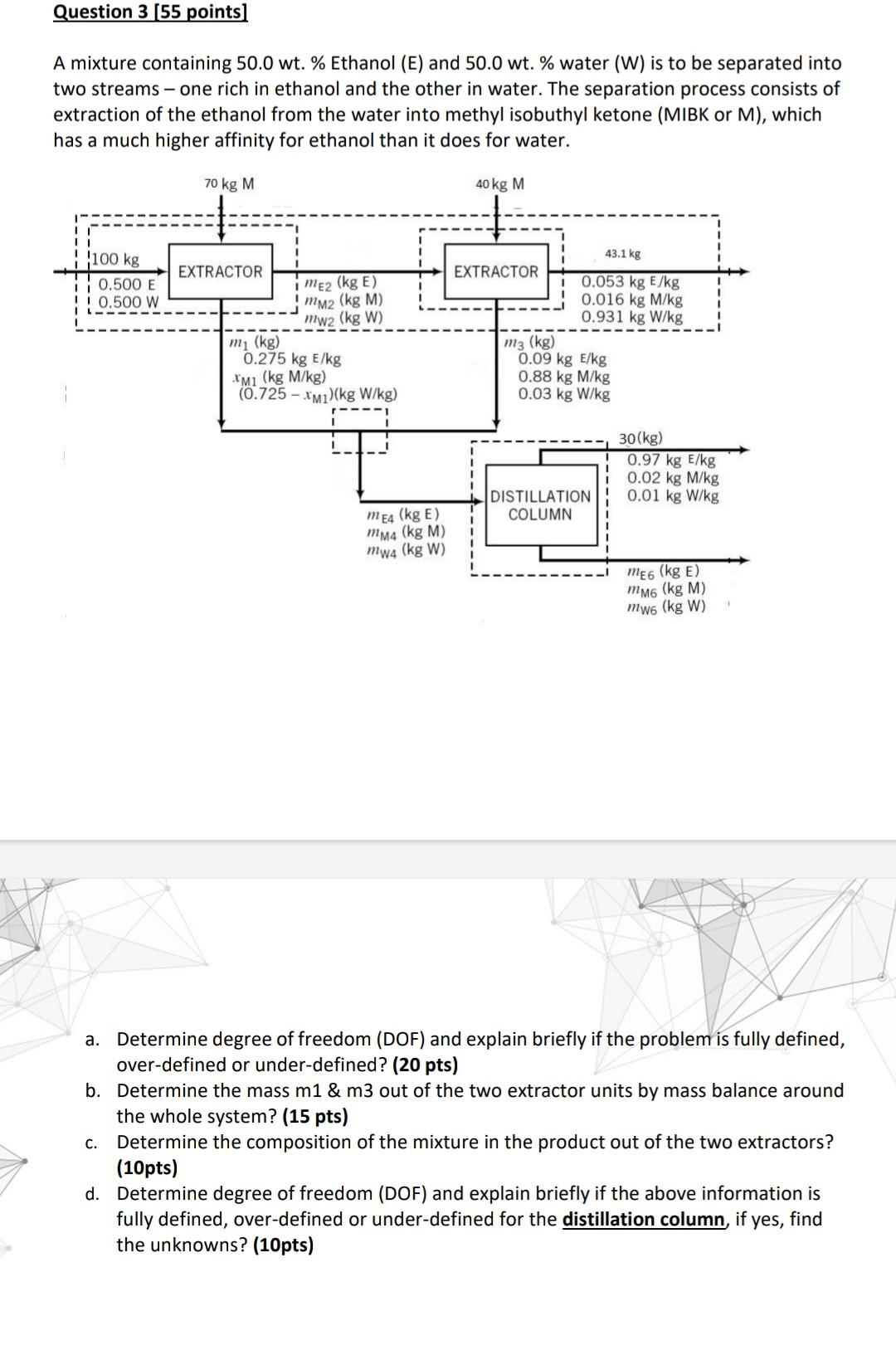
Question 3 (55 points) A mixture containing 50.0 wt. % Ethanol (E) and 50.0 wt. % water (W) is to be separated into two streams - one rich in ethanol and the other in water. The separation process consists of extraction of the ethanol from the water into methyl isobuthyl ketone (MIBK or M), which has a much higher affinity for ethanol than it does for water. 70 kg M 40 kg M 100 kg 43.1 kg EXTRACTOR EXTRACTOR 0.500 E ! 0.500 W mez (kg E) mm2 (kg M) mw2 (kg W) 0.053 kg E/kg 0.016 kg M/kg 0.931 kg W/kg mz (kg) mi (kg) 0.275 kg E/kg *M1 (kg M/kg) (0.725 - XM1)(kg W/kg) 0.09 kg E/kg 0.88 kg M/kg 0.03 kg W/kg 1 1 30(kg) 0.97 kg E/kg 0.02 kg M/kg 0.01 kg W/kg DISTILLATION COLUMN meg (kg E) mmg (kg M) mw4 (kg W) meg (kg E) Mm6 (kg M) mwo (kg W) C. a. Determine degree of freedom (DOF) and explain briefly if the problem is fully defined, over-defined or under-defined? (20 pts) b. Determine the mass m1 & m3 out of the two extractor units by mass balance around the whole system? (15 pts) Determine the composition of the mixture in the product out of the two extractors? (10pts) d. Determine degree of freedom (DOF) and explain briefly if the above information is fully defined, over-defined or under-defined for the distillation column if yes, find the unknowns? (10pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


