Question: Question 4 (2 points) Use the image to answer the following question. power supply 8 Sn(s) Inert C(s) Cd+ (aq) NO3(aq) electrolyte (solution) A student
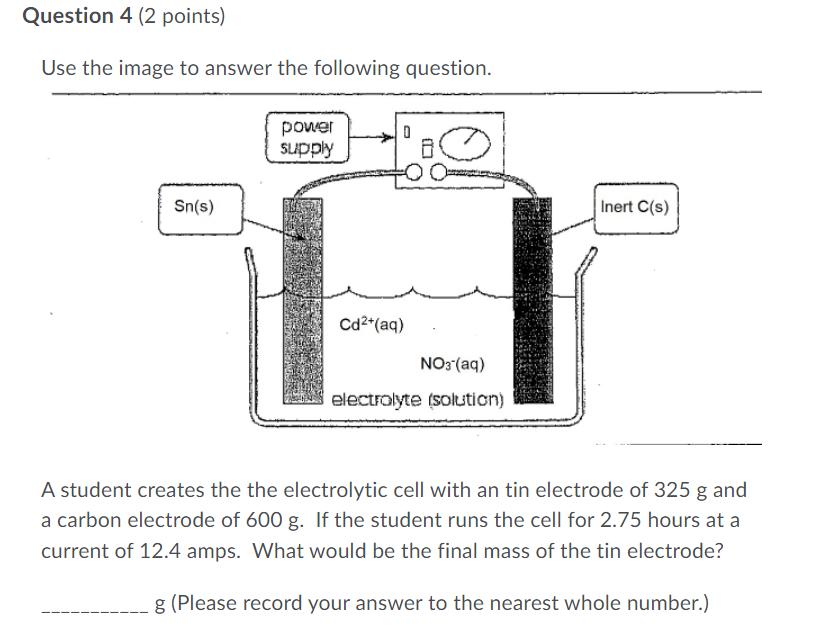
Question 4 (2 points) Use the image to answer the following question. power supply 8 Sn(s) Inert C(s) Cd+ (aq) NO3(aq) electrolyte (solution) A student creates the the electrolytic cell with an tin electrode of 325 g and a carbon electrode of 600 g. If the student runs the cell for 2.75 hours at a current of 12.4 amps. What would be the final mass of the tin electrode? g (Please record your answer to the nearest whole number.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


