QUESTION
Question #: ptsFor the elementary reversible liquidphase reaction :
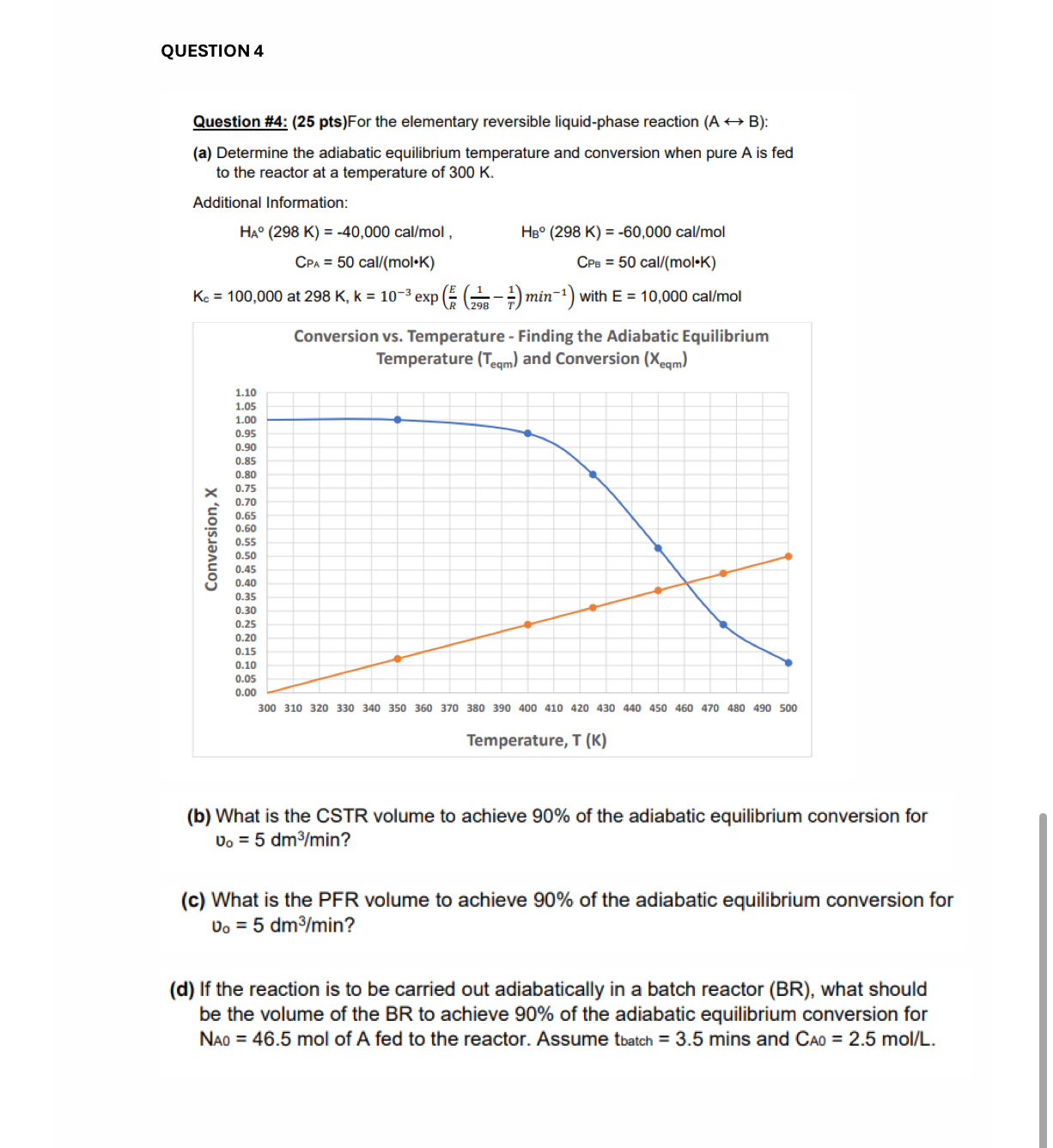
a Determine the adiabatic equilibrium temperature and conversion when pure is fed to the reactor at a temperature of
Additional Information:
exp with
at exp with
Conversion vs Temperature Finding the Adiabatic Equilibrium
b What is the CSTR volume to achieve of the adiabatic equilibrium conversion for
c What is the PFR volume to achieve of the adiabatic equilibrium conversion for
d If the reaction is to be carried out adiabatically in a batch reactor BR what should be the volume of the to achieve of the adiabatic equilibrium conversion for mol of A fed to the reactor. Assume tbatch mins and