Question: Question 5: Crystal growth kinetics (Total: 10 Marks) Batch crystal growth experiments were performed at a constant temperature of 10C to crystallise paracetamol from its
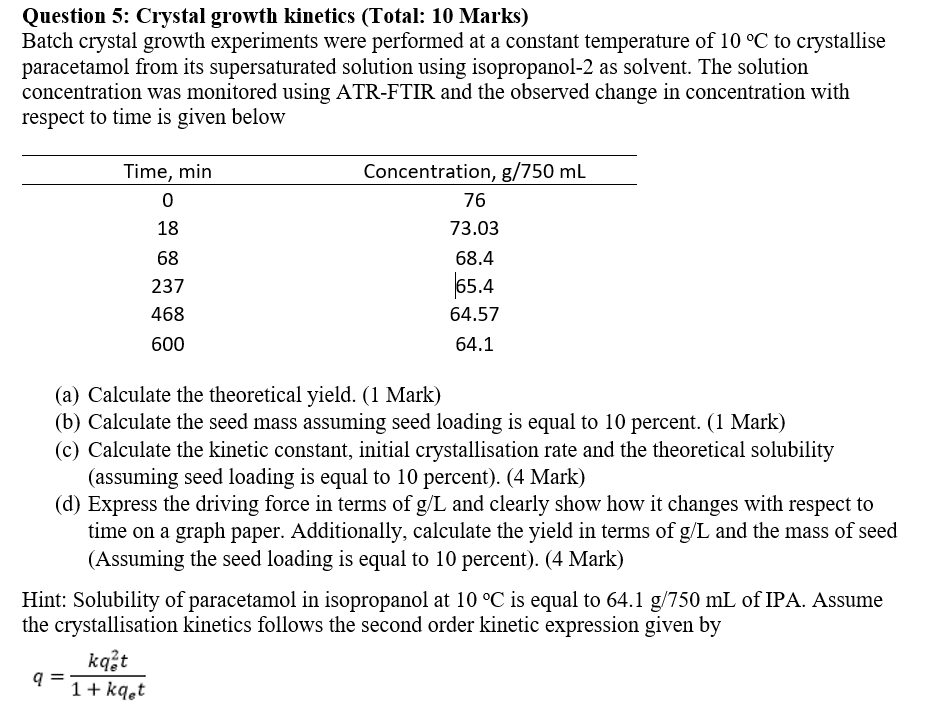
Question 5: Crystal growth kinetics (Total: 10 Marks) Batch crystal growth experiments were performed at a constant temperature of 10C to crystallise paracetamol from its supersaturated solution using isopropanol-2 as solvent. The solution concentration was monitored using ATR-FTIR and the observed change in concentration with respect to time is given below (a) Calculate the theoretical yield. (1 Mark) (b) Calculate the seed mass assuming seed loading is equal to 10 percent. (1 Mark) (c) Calculate the kinetic constant, initial crystallisation rate and the theoretical solubility (assuming seed loading is equal to 10 percent). (4 Mark) (d) Express the driving force in terms of g/L and clearly show how it changes with respect to time on a graph paper. Additionally, calculate the yield in terms of g/L and the mass of seed (Assuming the seed loading is equal to 10 percent). (4 Mark) Hint: Solubility of paracetamol in isopropanol at 10C is equal to 64.1g/750mL of IPA. Assume the crystallisation kinetics follows the second order kinetic expression given by q=1+kqetkqe2t Question 5: Crystal growth kinetics (Total: 10 Marks) Batch crystal growth experiments were performed at a constant temperature of 10C to crystallise paracetamol from its supersaturated solution using isopropanol-2 as solvent. The solution concentration was monitored using ATR-FTIR and the observed change in concentration with respect to time is given below (a) Calculate the theoretical yield. (1 Mark) (b) Calculate the seed mass assuming seed loading is equal to 10 percent. (1 Mark) (c) Calculate the kinetic constant, initial crystallisation rate and the theoretical solubility (assuming seed loading is equal to 10 percent). (4 Mark) (d) Express the driving force in terms of g/L and clearly show how it changes with respect to time on a graph paper. Additionally, calculate the yield in terms of g/L and the mass of seed (Assuming the seed loading is equal to 10 percent). (4 Mark) Hint: Solubility of paracetamol in isopropanol at 10C is equal to 64.1g/750mL of IPA. Assume the crystallisation kinetics follows the second order kinetic expression given by q=1+kqetkqe2t
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


