Question: Question 7 (CSTR) involves an elementary acid-catalyzed liquid phase reaction A B to be conducted adiabatically at constant pressure. The solution feed to the reactor

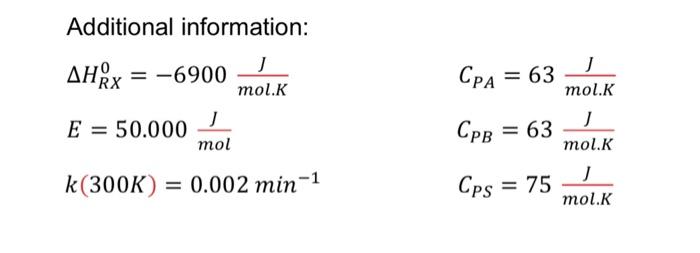
Question 7 (CSTR) involves an elementary acid-catalyzed liquid phase reaction A B to be conducted adiabatically at constant pressure. The solution feed to the reactor consists of an equimolar mixture of A and a solvent (contains catalyst) which enter the reactor at a total volumetric flow rate of 10 L/min with the 4 mol/L of A concentration The entering temperature is 300 K. Given the temperature range for the selected reaction is between 300 K to 500 K. Calculate: a) the conversion (X) and b) exit temperature (Text) that can be achieved in 1000 L and 2000 L of CSTR. calculation based on design; energy balance (adiabatic) and Arrhenius law equation. Choose reactor volume that is suitable for the process based on the conversion value obtained from both reactors. Plot graph on How the volume effects on the changes of X and Text. Additional information: : AHRx = -6900 ] mol.K = RX = CPA 63 mol.K = E = 50.000 mol CPB = 63 mol. Cps = 75 mol.K k(300K) = 0.002 min-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


