Question: Question 7 Identify the reducing agent in the following unbalanced redox equation: + NO Al AIO + NH Balance the following reduction-oxidation equation in acidic
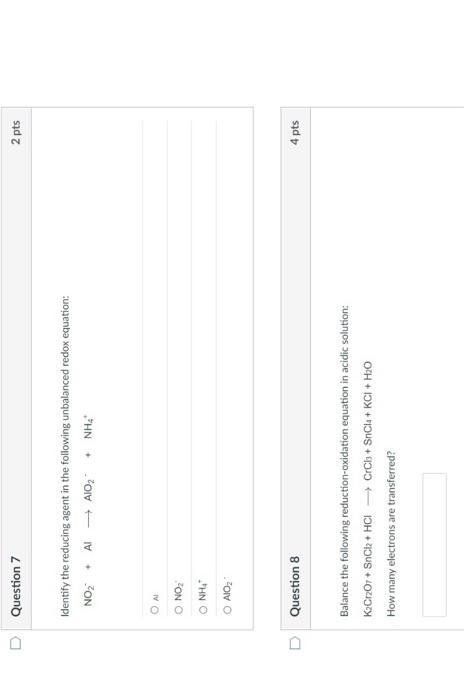
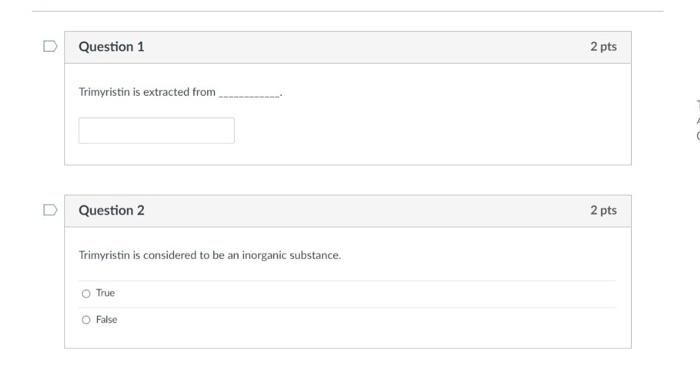
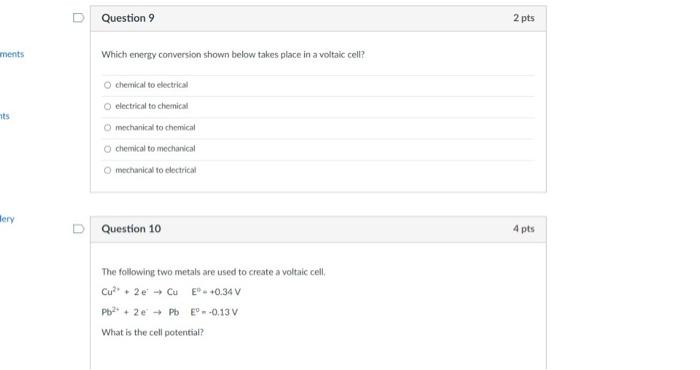
Question 7 Identify the reducing agent in the following unbalanced redox equation: + NO Al AIO + NH Balance the following reduction-oxidation equation in acidic solution: KCrO7+ SnCl + HCI CrCb + SnCla+KCI + HO How many electrons are transferred? O AL O NO I NH O AIO D Question 8 2 pts 4 pts Question 1 Trimyristin is extracted from Question 2 Trimyristin is considered to be an inorganic substance. True O False 2 pts 2 pts ments nts Mery Question 9 Which energy conversion shown below takes place in a voltaic cell? O chemical to electrical O electrical to chemical O mechanical to chemical O chemical to mechanical O mechanical to electrical Question 10 The following two metals are used to create a voltaic cell. Cu+ 2e Cu EP - +0.34 V Pb+ 2e Pb E--0.13 V What is the cell potential? 2 pts 4 pts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


