Question: question has starting materials. please answer b. What would silica TLC plates look like when this reaction is being monitored by TLC every 15 minutes
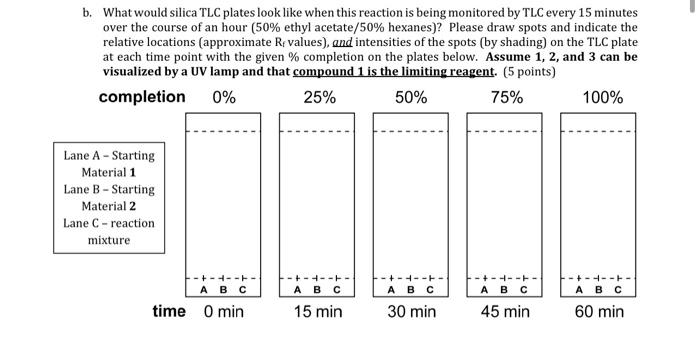
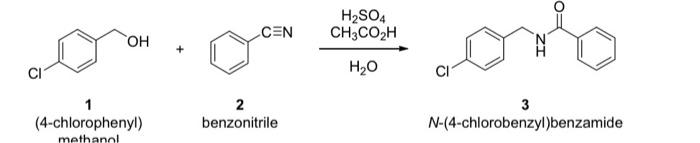
b. What would silica TLC plates look like when this reaction is being monitored by TLC every 15 minutes over the course of an hour (50% ethyl acetate/50% hexanes)? Please draw spots and indicate the relative locations (approximate R values), and intensities of the spots (by shading) on the TLC plate at each time point with the given % completion on the plates below. Assume 1, 2, and 3 can be visualized by a UV lamp and that compound 1 is the limiting reagent. (5 points) completion 0% 25% 50% 75% 100% Lane A - Starting Material 1 Lane B - Starting Material 2 Lane C-reaction mixture -+-+---- +-+-+-+ ---- time O min ------ ----- 60 min 15 min 30 min 45 min CEN H2SO4 CH3COH OH CI H2O CI (4-chlorophenyl) methanol 2 benzonitrile 3 N-(4-chlorobenzyl)benzamide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


