Question: QUESTION If U is considered a function of T and P, the natural heat capacity is neither Cy nor Cp, but rather the derivative
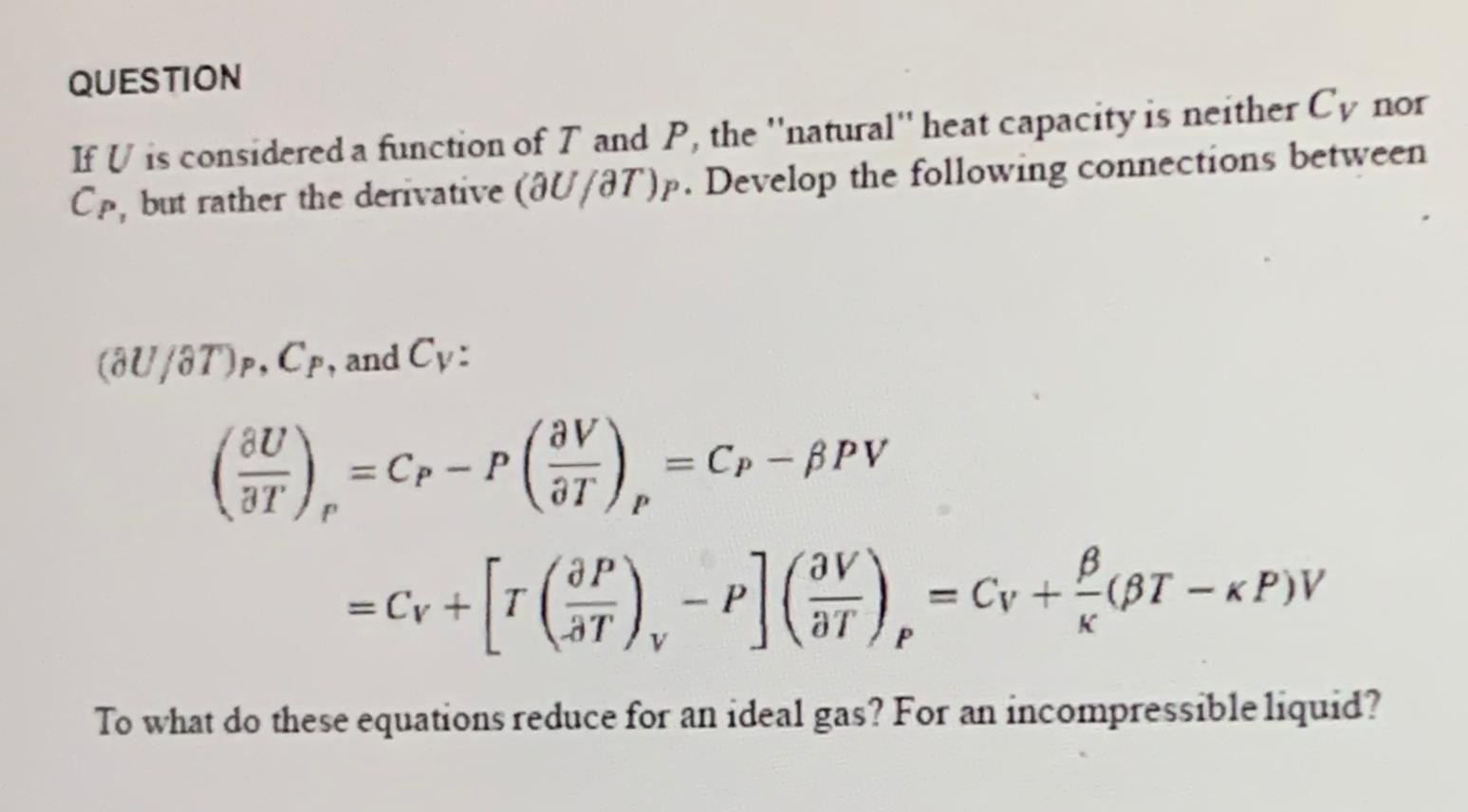
QUESTION If U is considered a function of T and P, the "natural" heat capacity is neither Cy nor Cp, but rather the derivative (aU/8T)p. Develop the following connections between (8U/8T)P, Cp, and Cy: (37), = C =Cp - P (37), = Cp - BPV Cv + [7 (7) - P] (r), = Cv + f (97 - x (F), (BT-KP)V = K P To what do these equations reduce for an ideal gas? For an incompressible liquid?
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
The relation between 37 Cp and C from the enthalpy definition H UPV UHPV By partial dif... View full answer

Get step-by-step solutions from verified subject matter experts


