Question: Question: Write the equilibrium-constant expressions and obtain numerical values for each constant in *(a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of
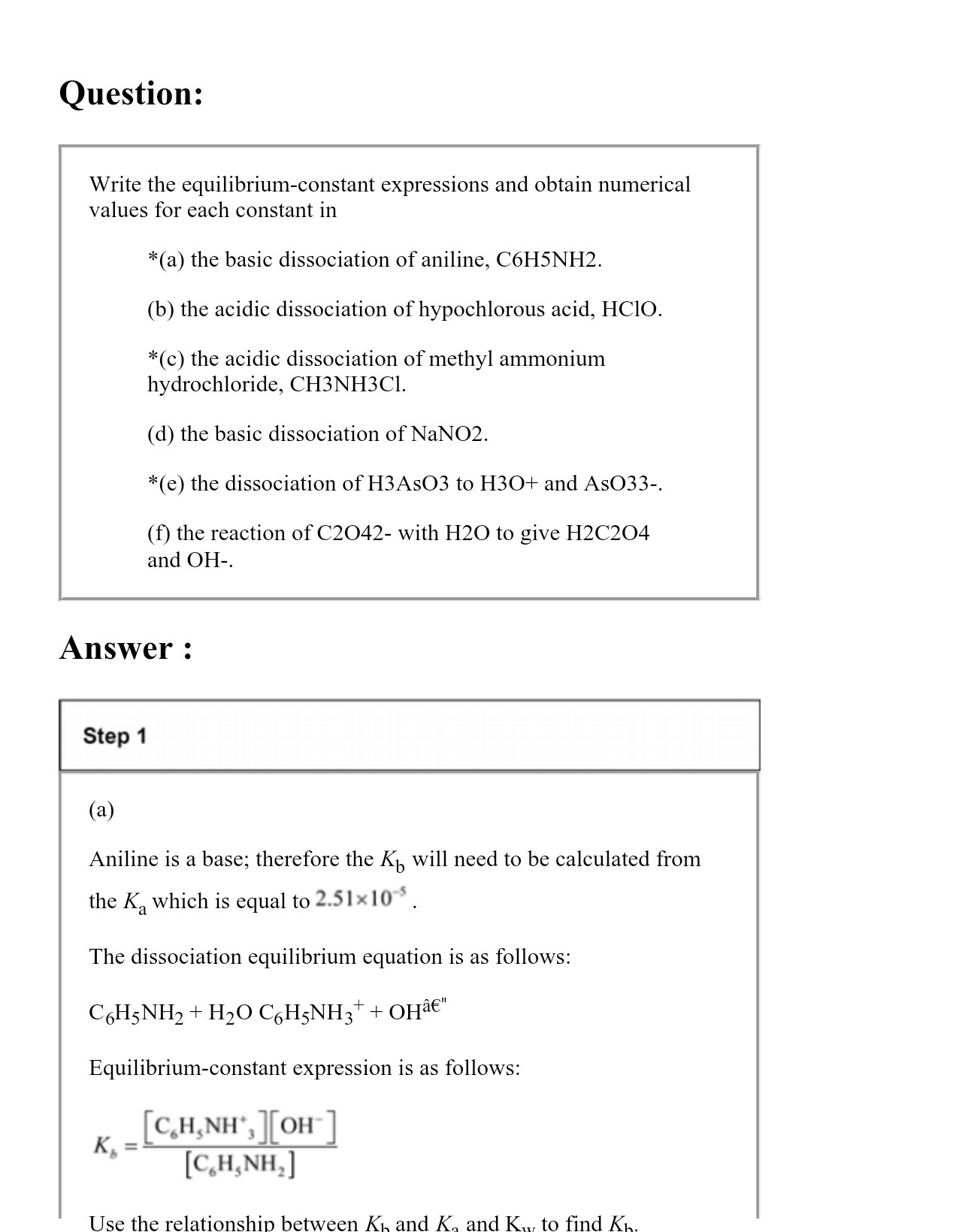
Question: Write the equilibrium-constant expressions and obtain numerical values for each constant in *(a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of hypochlorous acid, HCIO. *(c) the acidic dissociation of methyl ammonium hydrochloride, CH3NH3CI (d) the basic dissociation of NaNO2. *(e) the dissociation of H3As03 to H30+ and As033-. (f) the reaction of C2042- with H20 to give H2C204 and OH-. Answer : Step 1 (a) Aniline is a base; therefore the Kb will need to be calculated from the Ka which is equal to 2.51x10 5 . The dissociation equilibrium equation is as follows: CH5 NH2 + H20 CH5NH 3+ + OHaE" Equilibrium-constant expression is as follows: K, = [CH,NH, ][OH-] [C,H, NH,] Use the relationship between Ki and K, and K. to find Kh
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


