Question: R1 - LIQUID PHASE CHEMICAL REACTOR The elementary liquid phase reaction given below is carried out in a CSTR by isothermal operation. NaOH+EtOAckNaOAc+EtOH (A) (B)
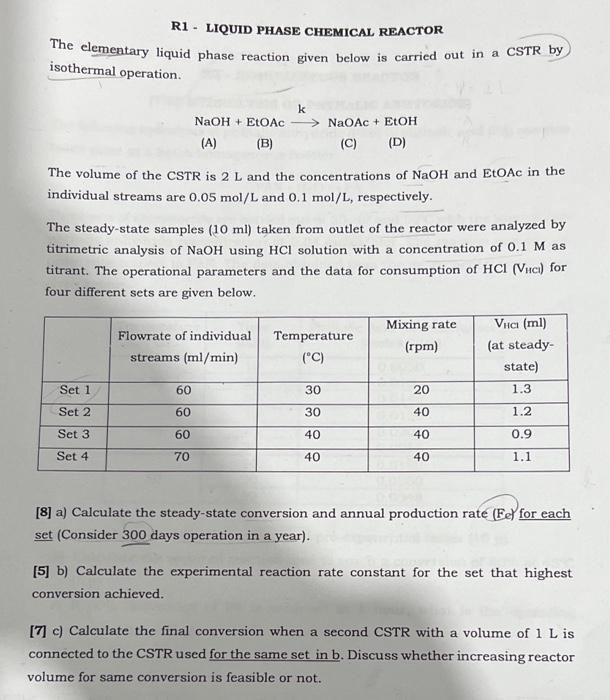
R1 - LIQUID PHASE CHEMICAL REACTOR The elementary liquid phase reaction given below is carried out in a CSTR by isothermal operation. NaOH+EtOAckNaOAc+EtOH (A) (B) (C) (D) The volume of the CSTR is 2L and the concentrations of NaOH and EtOAc in the individual streams are 0.05mol/L and 0.1mol/L, respectively. The steady-state samples (10ml) taken from outlet of the reactor were analyzed by titrimetric analysis of NaOH using HCl solution with a concentration of 0.1M as titrant. The operational parameters and the data for consumption of HCl(VHClH) for four different sets are given below. [8] a) Calculate the steady-state conversion and annual production rate (Fe) for each set (Consider 300 days operation in a year). [5] b) Calculate the experimental reaction rate constant for the set that highest conversion achieved. [7] c) Calculate the final conversion when a second CSTR with a volume of 1L is connected to the CSTR used for the same set in b. Discuss whether increasing reactor volume for same conversion is feasible or not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


