Question: Rate Law for Two Reactant Systems For the reaction A+ B the rate law can be written as: Rate = K[A][B] where k is the
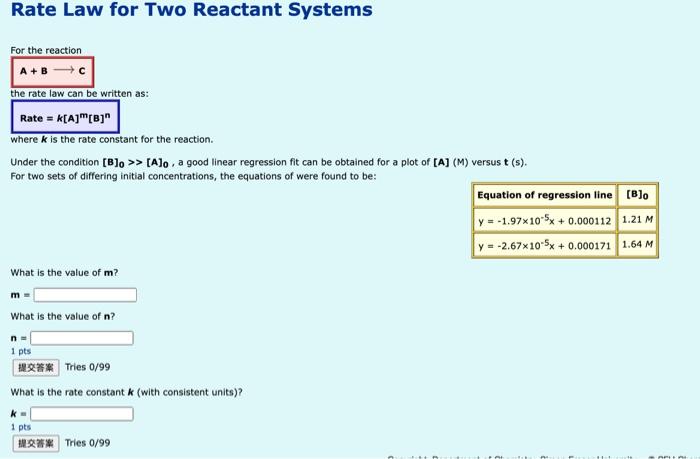
Rate Law for Two Reactant Systems For the reaction A+ B the rate law can be written as: Rate = K[A][B]" where k is the rate constant for the reaction. Under the condition [B]. >> [A]o, a good linear regression fit can be obtained for a plot of [A] (M) versust (s). For two sets of differing initial concentrations, the equations of were found to be: Equation of regression line [B] y = -1.97x10-5x + 0.000112 1.21 M y = -2.67x10-SX +0.000171 1.64 M What is the value of m? m What is the value of n? n- 1 pts HER** Tries 0/99 What is the rate constant k (with consistent units)? 1 pts M.:28 Tres 0/99
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


