Question: Reactant A and reactant B are fed into a reactor as shown. The reaction is A+BC The product, C, is separated from the remaining non-reacted
Reactant A and reactant B are fed into a reactor as shown. The reaction is A+BC The product, C, is separated from the remaining non-reacted amounts of A and B in distillation column 1. The non-reacted substances are again distilled to separate the two reactants, and one of the streams is recycled as shown. What is the molar flow rate of recycle stream 7?
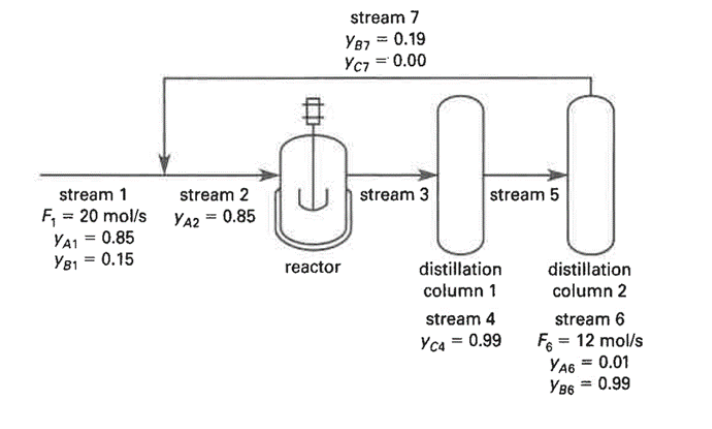
stream 1 F = 20 mol/s YA1 = 0.85 Y81 = 0.15 stream 2 YA2 = 0.85 stream 7 YB7 = 0.19 YC7 = 0.00 stream 3 reactor -0-0 stream 5 distillation distillation column 1 column 2 stream 4 stream 6 Yc4 = 0.99 F = 12 mol/s YA6 = 0.01 YB6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


