Question: Reactant A reacts with B through two simultaneous reactions to form two products, one of which is the desired product D and one of which
Reactant A reacts with B through two simultaneous reactions to form two products, one of which is the desired product D and one of which is the undesired product U.
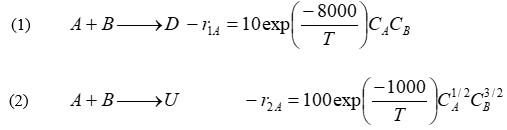
How and under what conditions (i.e. type(s) of reactor(s), temperature, concentrations) should the reaction be carried out to maximize the selectivity of D, having an inlet concentration of A = 0.4 M and of B = 0.1 M and a volumetric flow rate of 2.0 L/s?
(1) A+BDr1A=10exp(T8000)CACB (2) A+BUr2A=100exp(T1000)CA1/2CB3/2 (1) A+BDr1A=10exp(T8000)CACB (2) A+BUr2A=100exp(T1000)CA1/2CB3/2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


