Question: Reaction 1 - prepare the reagent table for the scenario below. (make sure to indicate if each chemical is a reagent, solvent, or reactant. show
Reaction 1 - prepare the reagent table for the scenario below. (make sure to indicate if each chemical is a reagent, solvent, or reactant. show all your calculations, including all units, and references.)
Weigh out 0.36 g of magnesium turnings, then place it in RBF. In separatory funnel, add 1.5 ml bromobenzene and 15 ml ether and shake it to make the solution homogeneous. Open the stopcock and let down enough of ether solution into flask to cover all the magnesium. Start stirring. Turn on cooling water of the condenser, then heat the reaction mixture using steaming bath, until the reaction mixture becomes brown or chalky and the spontaneous reflux continues. (Turn off the steam bath and check if reaction reflux by itself without external heating resource.) When the Grignard reaction initiated, add the rest of the ether solution into RBF drop wise over 10 min, with low steam. When addition is finished, reflux the reaction mixture for additional 30 min. In addition, funnel, add 1.83 g of 4-chlorobenzaldehyde and 15 mL of ether, then shake it well. Stop heating, add the solution drop wise to flask over 10 min. When addition is finished, continue to heat the solution for 5 min.
Work up
In 250 mL beaker, place 30 mL of 1.8 M H2SO4 and ice to make 60 mL acidic solution. Pour the reaction mixture into the beaker while stirring using stir rod. Use a few mL of water and ether to rinse the RBF, but leave behind the unreacted Mg.
Then transfer the liquid to separation funnel, draw off the aqueous layer. Wash the organic phase with 15 mL of 5% NaOH solution, then with 15 mL of Sat. NaCl solution.
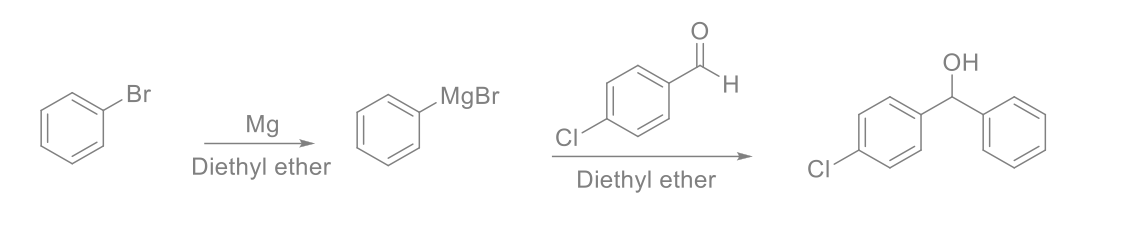
OH Br MgBr Mg Diethyl ether Diethyl ether
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


