Question: Reaction 2 - prepare the reagent table for the scenario below. (make sure to indicate if each chemical is a reagent, solvent, or reactant. show
Reaction 2 - prepare the reagent table for the scenario below. (make sure to indicate if each chemical is a reagent, solvent, or reactant. show all your calculations, including all units, and references.)
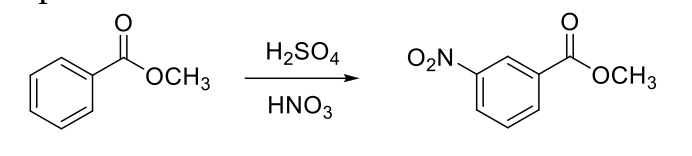
Place 25 mL RBF with a small magnetic stir bar on ice bath which is on a stirrer. Place 8 mL of H2SO4 in the RBF and cautiously add 1 mL of HNO3.
Cool the mixture to get less than 10 oC if placed inside RBF or less than 5 oC if placed inside beaker Weigh the vial with compound (in presence of your TA) and add 3.0 g methyl benzoate very slowly 4-5 drops at a time with pipette. Mix thoroughly after adding each portion. After adding methyl benzoate, record the weight of empty vial (in presence of your TA). (limiting reagent).
Start stirring at 10-15 oC for 10 mins. Remove the ice bath and stir 15 mins more. Place 20 mL of water and few pieces of ice in a beaker and place this beaker in another ice bath.
Pour the nitration solution in the beaker, rinse the flask with little amount of ice-cold water and stir the solution till the initial turbidity dissipated. wait for complete precipitation.
Please let me know if there is something wrong, or need some more information.
Thank you so much.
1 H2SO4 O,N. OCH3 3 HNO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


