Question: reaction takes place in a porous solid catalyst. Develop a selectivity expression for this reaction. Explain how increasing CB affects selectivity. R is the unwanted
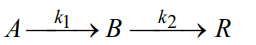
reaction takes place in a porous solid catalyst. Develop a selectivity expression for this reaction. Explain how increasing CB affects selectivity. R is the unwanted product. Also do your calculations by taking the dimensions of k1 and k2 (1/time) and k1>k2.
How does the pore structure of a porous solid catalyst affect the selectivity in this reaction?
Ak1Bk2R
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


