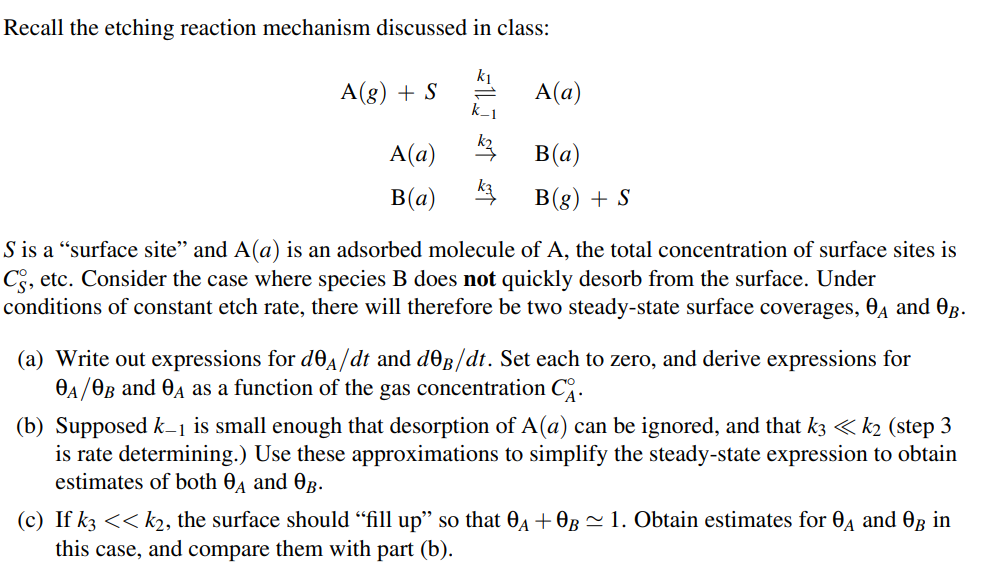
Question: Recall the etching reaction mechanism discussed in class: A ( g ) + S , k 1 , A ( a ) A ( a
Recall the etching reaction mechanism discussed in class:
is a "surface site" and is an adsorbed molecule of the total concentration of surface sites is
etc. Consider the case where species B does not quickly desorb from the surface. Under
conditions of constant etch rate, there will therefore be two steadystate surface coverages, and
a Write out expressions for and Set each to zero, and derive expressions for
and as a function of the gas concentration
b Supposed is small enough that desorption of can be ignored, and that step
is rate determining. Use these approximations to simplify the steadystate expression to obtain
estimates of both and
c If the surface should "fill that Obtain estimates for and
this case, and compare them with part
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


