Question: Refer to the table of standard reduction potentials, E red, provided below. In an acidic solution, copper(I) ion is oxidized to copper(II) ion by the
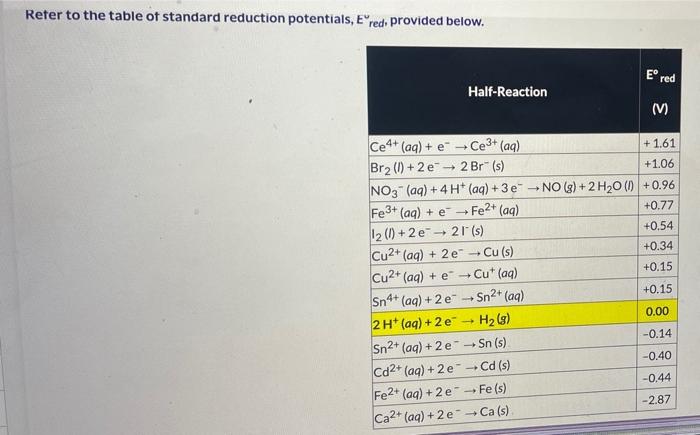

Refer to the table of standard reduction potentials, E red, provided below. In an acidic solution, copper(I) ion is oxidized to copper(II) ion by the nitrate ion. 3Cu+(aq)+NO3(aq)+4H+(aq)NO(g)+3Cu2+(aq)+2H2O(l) Part A: Using the E red values provided, calculate the standard cell potential, E cell, (in V) for this reaction. Round your answer to TWO places past the decimal. If your answer is negative, include the sign. Ecell= Part B: Use the rounded value of E cell from Part A to calculate the standard free energy, G, (in kJ ) of the reaction at 298K Round your answer to the nearest whole number. If your answer is negative, include the sign. G= Part C: Use the rounded value of E cell from Part A to calculate the equilibrium constant, K, at 298K. Round your answer to ONE place past the decimal in scientific notation. If your answer is negative, include the sign
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


