Question: References Use the References to access important values if needed for this question. For the gas phase decomposition of ethyl chloroformate, clcooC2H5-C2H5Cl + CO2 the
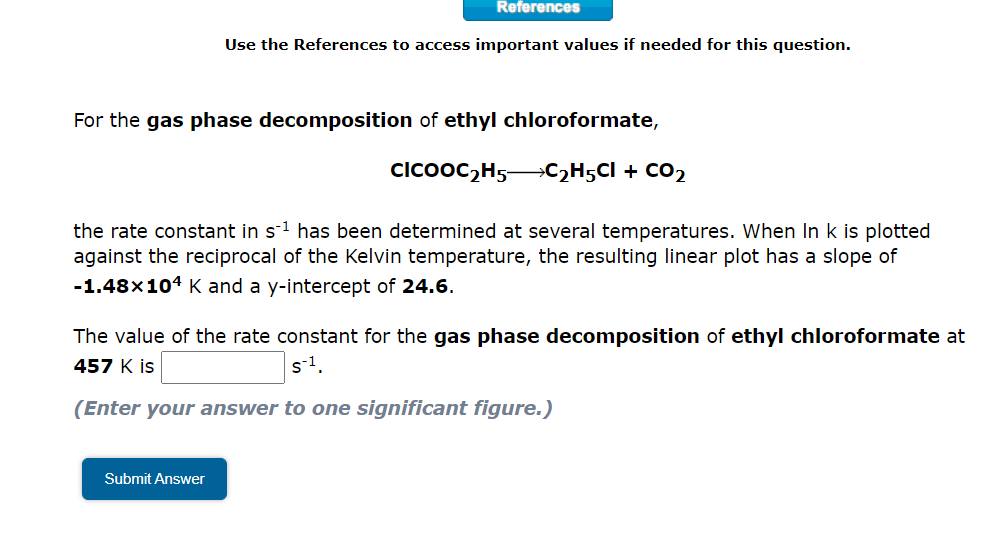
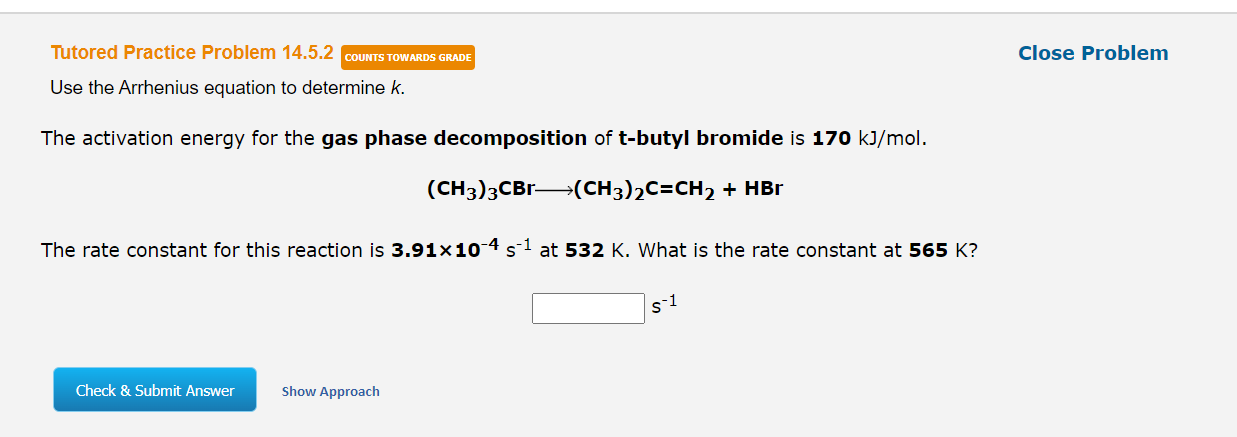
References Use the References to access important values if needed for this question. For the gas phase decomposition of ethyl chloroformate, clcooC2H5-C2H5Cl + CO2 the rate constant in s-1 has been determined at several temperatures. When In k is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -1.48x104 K and a y-intercept of 24.6. The value of the rate constant for the gas phase decomposition of ethyl chloroformate at 457 K is s1. (Enter your answer to one significant figure.) Submit Answer Tutored Practice Problem 14.5.2 COUNTS TOWARDS GRADE Close Problem Use the Arrhenius equation to determine k. The activation energy for the gas phase decomposition of t-butyl bromide is 170 kJ/mol. (CH3)3CBr(CH3)2C=CH2 + HBr The rate constant for this reaction is 3.91x10-4 5-1 at 532 K. What is the rate constant at 565 K? s-1 Check & Submit Answer Show Approach
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


