Question: Repeat the example question in the lecture Synthesis of Metals regarding with the reduction of Cr2O3 to Cr at 1400 C (1673 K) in a
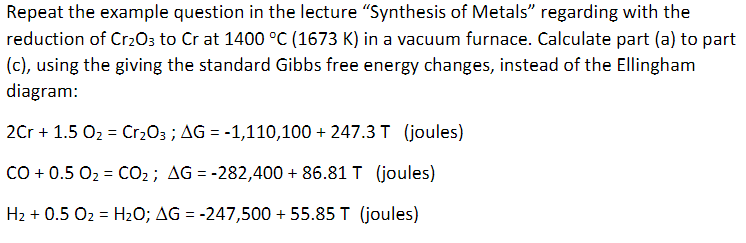
Repeat the example question in the lecture Synthesis of Metals regarding with the reduction of Cr2O3 to Cr at 1400 C (1673 K) in a vacuum furnace. Calculate part (a) to part (c), using the giving the standard Gibbs free energy changes, instead of the Ellingham diagram: 2Cr + 1.5 O2 = Cr2O3 ; AG = -1,110,100 + 247.3T (joules) = CO+ 0.5 Oz = CO2; AG = -282,400 + 86.81 T (joules) H2 + 0.5 Oz = H2O; AG = -247,500 + 55.85 T (joules) - =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


