Question: Ribose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in principle, could contain either a four-membered, five-membered, or six-membered ring. To
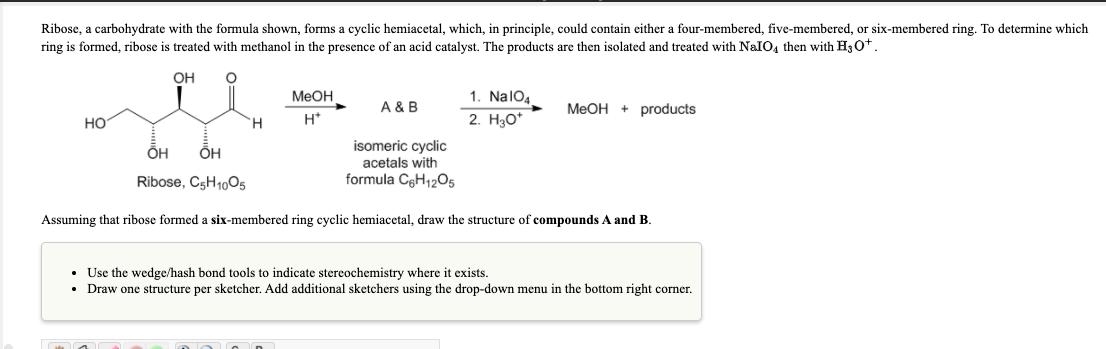
Ribose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in principle, could contain either a four-membered, five-membered, or six-membered ring. To determine which ring is formed, ribose is treated with methanol in the presence of an acid catalyst. The products are then isolated and treated with NaIO4 then with H30*. OH MeOH 1. NalO4 A & B MeOH + products H. H* 2. H30* H isomeric cyclic acetals with Ribose, C5H1005 formula CeH12O5 Assuming that ribose formed a six-membered ring cyclic hemiacetal, draw the structure of compounds A and B. Use the wedge/hash bond tools to indicate stereochemistry where it exists. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


