Question: Salt Diffusion Through a Reverse Osmosis Membrane Assume you have a thin membrane film to be used for reverse osmosis: water purification from salt water.
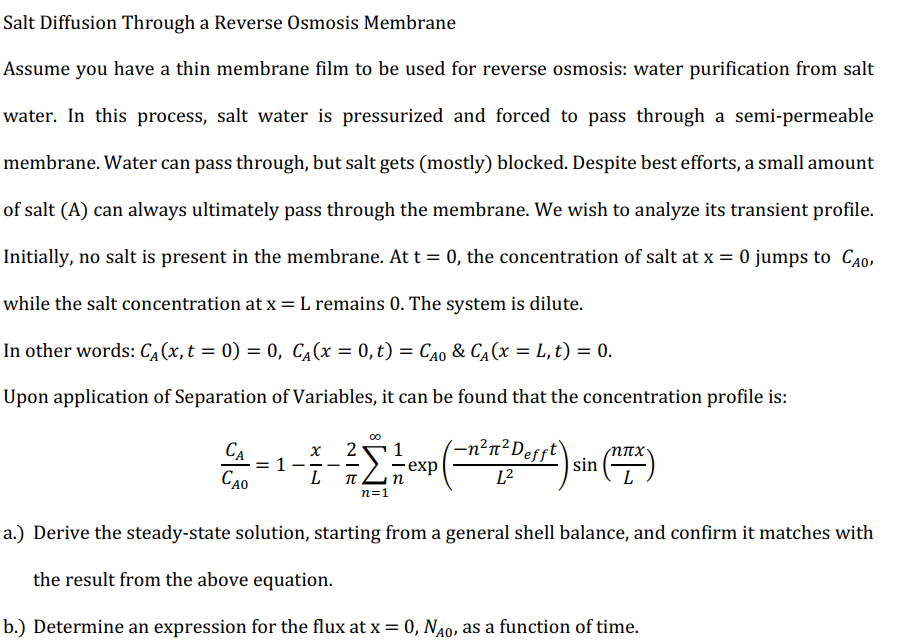
Salt Diffusion Through a Reverse Osmosis Membrane Assume you have a thin membrane film to be used for reverse osmosis: water purification from salt water. In this process, salt water is pressurized and forced to pass through a semi-permeable membrane. Water can pass through, but salt gets (mostly) blocked. Despite best efforts, a small amount of salt (A) can always ultimately pass through the membrane. We wish to analyze its transient profile. Initially, no salt is present in the membrane. At t=0, the concentration of salt at x=0 jumps to CA0, while the salt concentration at x=L remains 0 . The system is dilute. In other words: CA(x,t=0)=0,CA(x=0,t)=CA0&CA(x=L,t)=0. Upon application of Separation of Variables, it can be found that the concentration profile is: CA0CA=1Lx2n=1n1exp(L2n22Defft)sin(Lnx) a.) Derive the steady-state solution, starting from a general shell balance, and confirm it matches with the result from the above equation. b.) Determine an expression for the flux at x=0,NA0, as a function of time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


