Question: Same data with several 3 questions (please show solution) Consider a water sample with the following ion concentrations. Assume no particulate form exists in the
Same data with several 3 questions (please show solution)
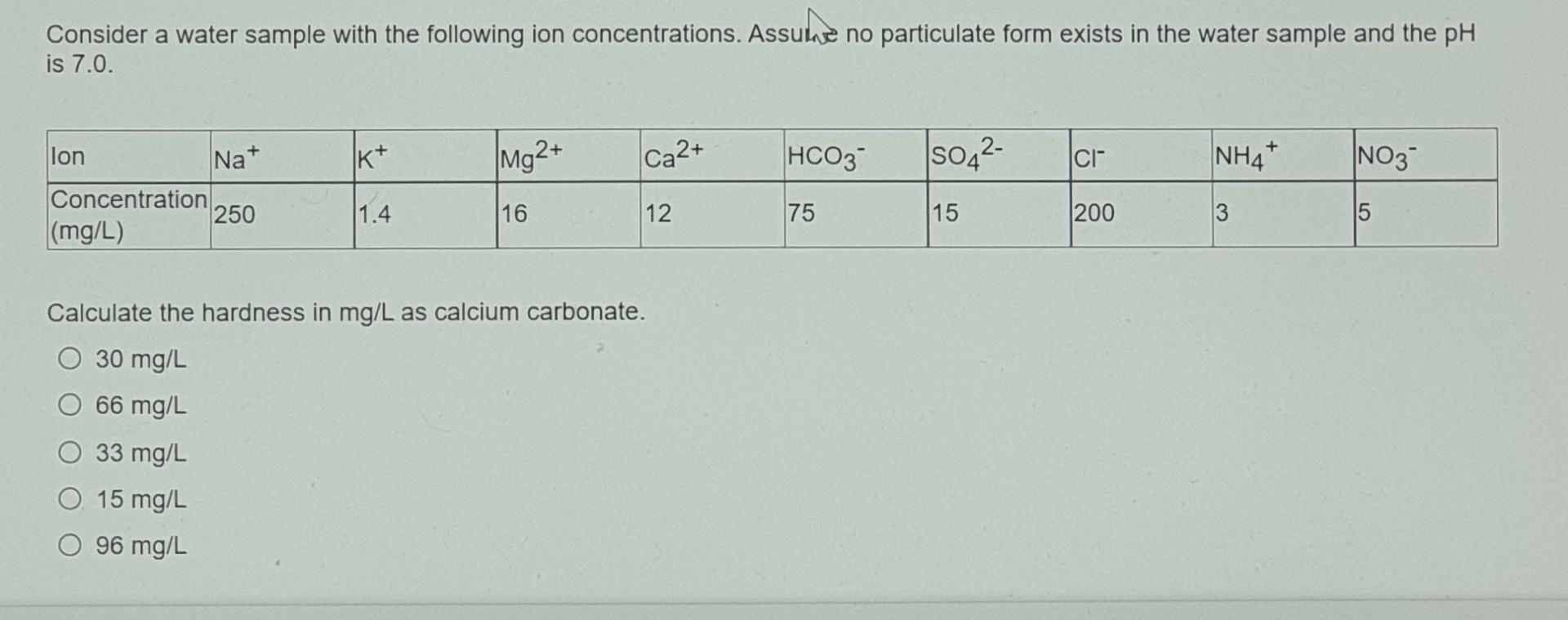


Consider a water sample with the following ion concentrations. Assume no particulate form exists in the water sample and the pH is 7.0. K+ Mg2+ Ca2+ HCO3- [s042- ci NH4+ NO3 lon Na+ Concentration 250 (mg/L) 1.4 16 12 75 15 200 3 5 Calculate the hardness in mg/L as calcium carbonate. O 30 mg/L 66 mg/L O 33 mg/L O 15 mg/L O 96 mg/L Calculate the concentration of the total dissolved solids in mg/L. O 251.4 mg/L O 208 mg/L 250 mg/L O 278 mg/L O 577.4 mg/L Calculate the specific conductivity from the following empirical correlation: TDS = 0.6 x specific conductivity (uS/cm) O 962.3 S/cm O 346.7 uS/cm O 416.7 S/cm 463.3 ps/cm O 419 uS/cm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


