Question: *same problem Question 3 (20) Ammonia is oxidized to form nitric oxide (for synthesis of HNO3) by the following reaction: 4NH3 + 502 4N0 +
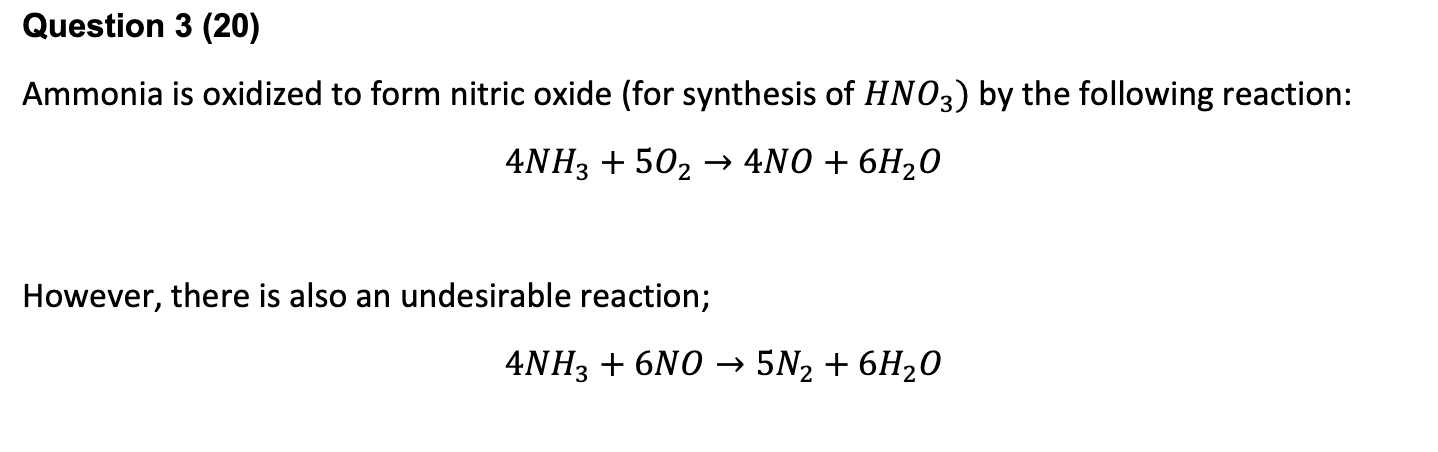
 *same problem
*same problem
Question 3 (20) Ammonia is oxidized to form nitric oxide (for synthesis of HNO3) by the following reaction: 4NH3 + 502 4N0 + 6H2O However, there is also an undesirable reaction; 4NH3 + 6NO 5N2 + 6H2O A stream consisting of 7 kmol/min ammonia and 10 kmol/min oxygen is fed to a reactor. Let $1 denote the extent of the desired reaction and $2 the extent of the second (undesirable) reaction. Label the product stream nnh3, No2, nno, N420, nnz (all in kmol/min). If 60% of the ammonia is converted (desired and undesired reactions) and nno 5nn2, find the following: = 1.The extent of each reaction and the gas composition. (12) 2. The yield of NO. (4) 3. The selectivity of NO with respect to N2. (4)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


