Question: Second order irreversible reaction take place in a batch type reactor. Initial concentration of A is 5mol/L. Calculate the time needed to reach 80% conversion
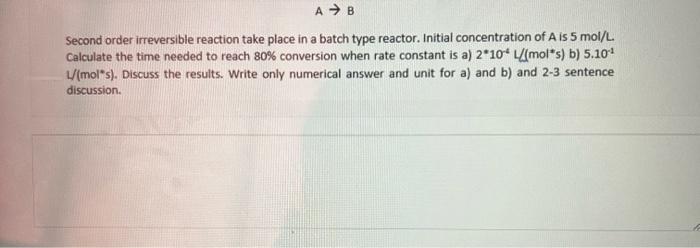
Second order irreversible reaction take place in a batch type reactor. Initial concentration of A is 5mol/L. Calculate the time needed to reach 80% conversion when rate constant is a) 2104L/(mols) b) 5.101 L( mol s). Discuss the results. Write only numerical answer and unit for a) and b) and 2-3 sentence discussion. Second order irreversible reaction take place in a batch type reactor. Initial concentration of A is 5mol/L. Calculate the time needed to reach 80% conversion when rate constant is a) 2104L/(mols) b) 5.101 L( mol s). Discuss the results. Write only numerical answer and unit for a) and b) and 2-3 sentence discussion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


