Question: second time asking could really use the help (So3 is not .5 , air is not 3.1, and so2 is not .7) if possible please
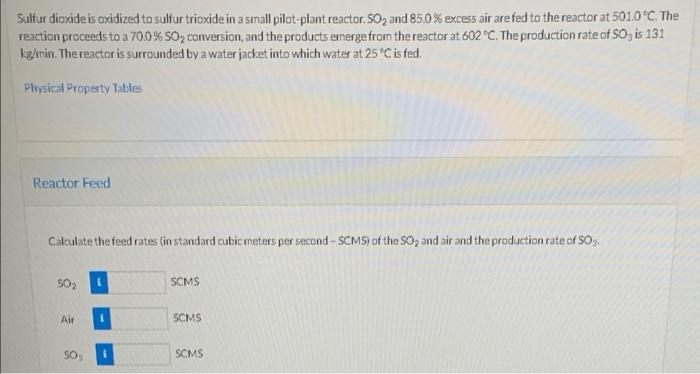
Sulfur dioxide is oxidized to sulfur trioxide in a small pilot-plant reactor. 50, and 85.0% excess air are fed to the reactor at 501.0C. The reaction proceeds to a 70.0% 50, conversion, and the products emerge from the reactor at 602 "C. The production rate of SO, is 131 kg/min. The reactor is surrounded by a water jacket into which water at 25C is fed. Physical Property Tables Reactor Feed Calculate the feed rates (in standard cubic meters per second-SCMS) of the SO2 and air and the production rate of S03. SO, SCMS Air SCMS SO SCMS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


