Question: Separation process. Urgent. please help me with full handwritting solution. Please dont take answers from other source. Thank you A waste acidic stream contains copper
Separation process. Urgent. please help me with full handwritting solution. Please dont take answers from other source. Thank you
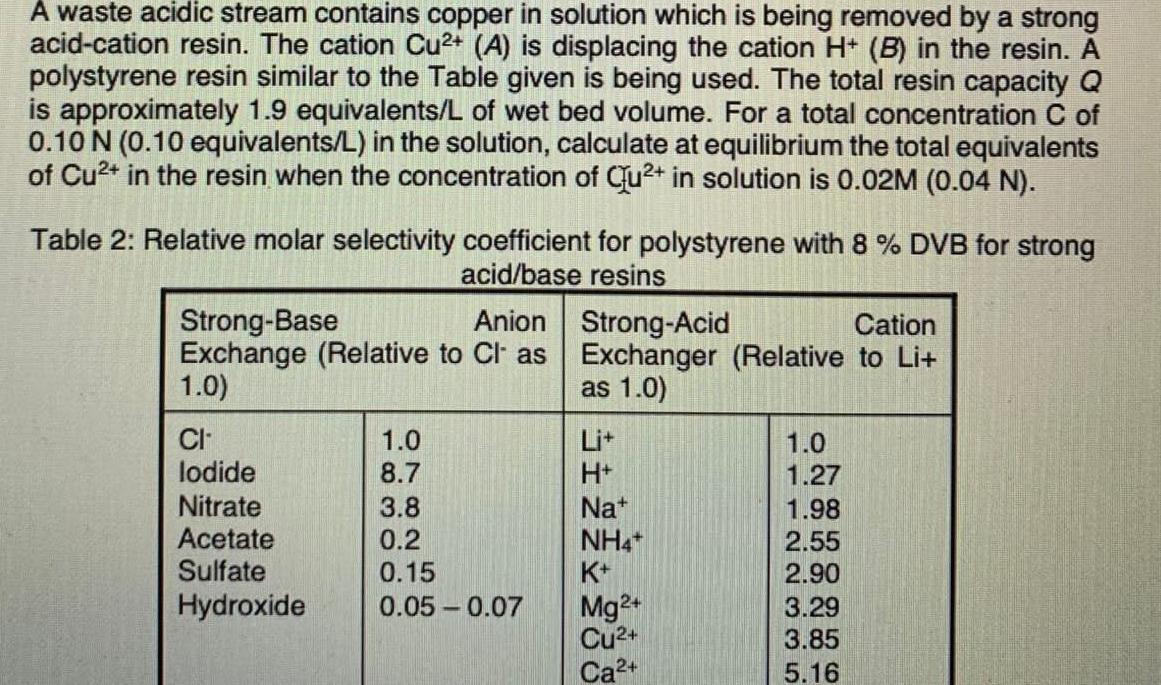
A waste acidic stream contains copper in solution which is being removed by a strong acid-cation resin. The cation Cu2+ (A) is displacing the cation H+ (B) in the resin. A polystyrene resin similar to the Table given is being used. The total resin capacity Q is approximately 1.9 equivalents/L of wet bed volume. For a total concentration C of 0.10 N (0.10 equivalents/L) in the solution, calculate at equilibrium the total equivalents of Cu2+ in the resin when the concentration of Cu2+ in solution is 0.02M (0.04 N). Table 2: Relative molar selectivity coefficient for polystyrene with 8 % DVB for strong acid/base resins Strong-Base Anion Strong-Acid Cation Exchange (Relative to Cl' as Exchanger (Relative to Li+ 1.0) as 1.0) CI lodide Nitrate Acetate Sulfate Hydroxide 1.0 8.7 3.8 0.2 0.15 0.05 -0.07 H+ Nat NH4+ K+ 1.0 1.27 1.98 2.55 2.90 3.29 3.85 5.16 Mg2+ Cu2+ Ca2+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


