Question: show all the working steps and answer in measurable quantities. The elementary steps for CO oxidation on Pt catalyst (*): CO(g) + * = CO*
show all the working steps and answer in measurable quantities.
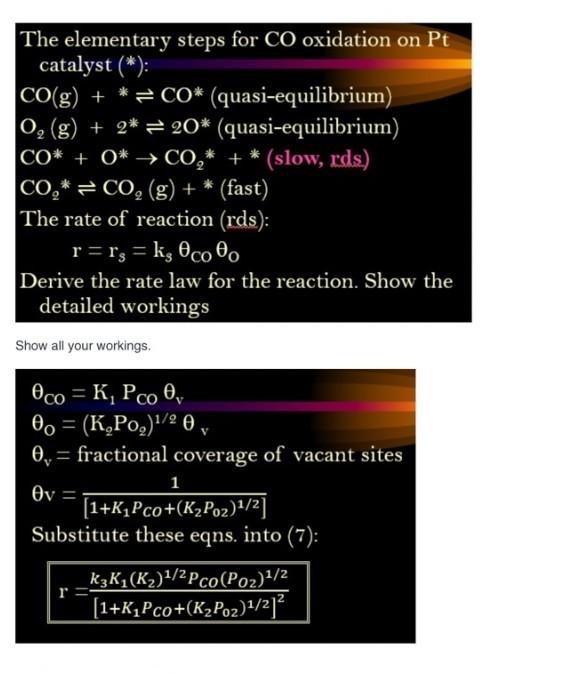
The elementary steps for CO oxidation on Pt catalyst (*): CO(g) + * = CO* (quasi-equilibrium) 0, (g) + 2* = 20* (quasi-equilibrium) CO* + 0* CO,* + * (slow, rds) CO,* = CO, (g) + * (fast) The rate of reaction (rds): r=rg = kg Oco Derive the rate law for the reaction. Show the detailed workings Show all your workings 1 = Oco = K, Pco 0, 0o = (K,Po,)1/2 0 0,= fractional coverage of vacant sites v (1+K_Pco+(K_Poz)1/2] Substitute these eqns into (7): k3K_(K)1/2 Pco (Poz)1/2 [1+KP co+(KzPoz)1/212 r
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


