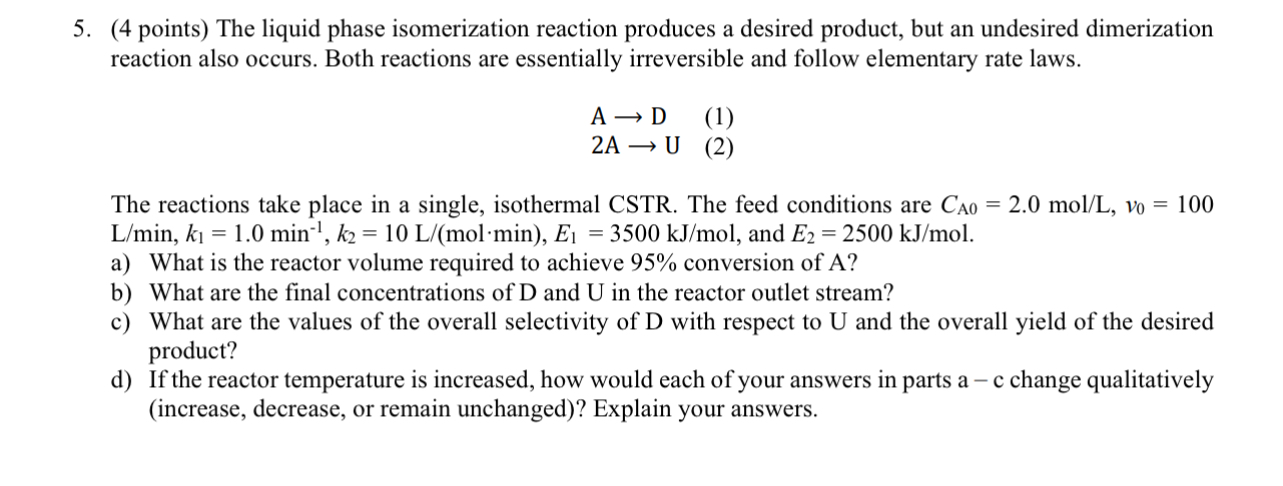
Question: SHOW POLYMATH CODE!!! The liquid phase isomerization reaction produces a desired product, but an undesired dimerization reaction also occurs. Both reactions are essentially irreversible and
SHOW POLYMATH CODE!!! The liquid phase isomerization reaction produces a desired product, but an undesired dimerization reaction also occurs. Both reactions are essentially irreversible and follow elementary rate laws.
The reactions take place in a single, isothermal CSTR The feed conditions are and
a What is the reactor volume required to achieve conversion of
b What are the final concentrations of and in the reactor outlet stream?
c What are the values of the overall selectivity of with respect to and the overall yield of the desired product?
d If the reactor temperature is increased, how would each of your answers in parts a change qualitatively increase decrease, or remain unchanged Explain your answers.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


