Question: show work and solve Use the References to access important values if needed for this question. The following initial rate data are for the reaction
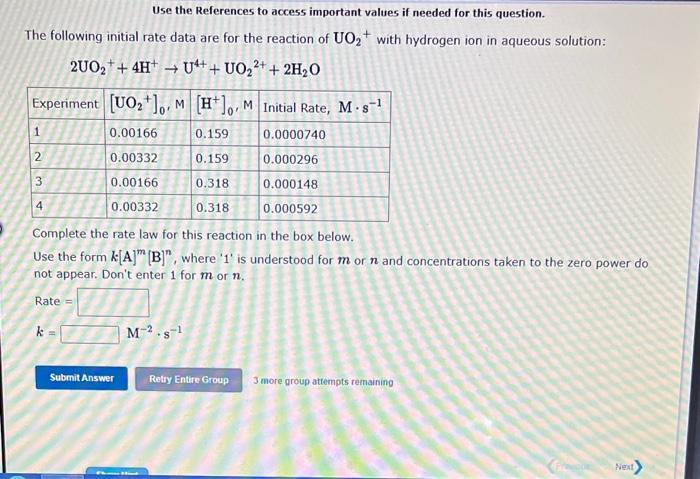
Use the References to access important values if needed for this question. The following initial rate data are for the reaction of UO2+ with hydrogen ion in aqueous solution: 2UO2++4H+U4++UO22++2H2O Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n, where ' 1 ' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n. Rate = k=M2s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


