Question: show work and solve Use the References to access important values if needed for this question. The decomposition of hydrogen iodide on a gold surface
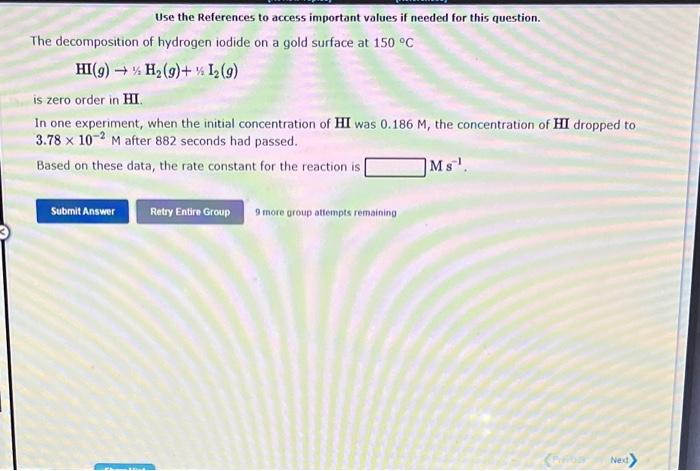
Use the References to access important values if needed for this question. The decomposition of hydrogen iodide on a gold surface at 150C HI(g)1/2H2(g)+1/2I2(g) is zero order in HI. In one experiment, when the initial concentration of HI was 0.186M, the concentration of HI dropped to 3.78102M after 882 seconds had passed. Based on these data, the rate constant for the reaction is Ms1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


