Question: Use the References to access important values if needed for this question. In a study of the decomposition of hydrogen iodide on a gold surface
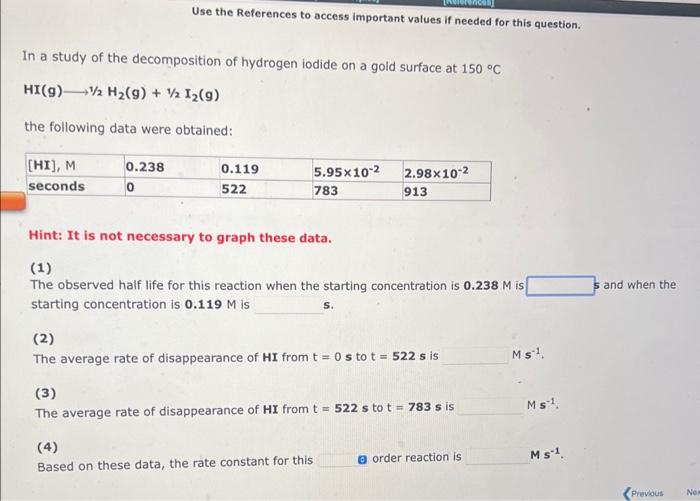
Use the References to access important values if needed for this question. In a study of the decomposition of hydrogen iodide on a gold surface at 150C HI(g)1/2H2(g)+1/2I2(g) the following data were obtained: Hint: It is not necessary to graph these data. (1) The observed haif life for this reaction when the starting concentration is 0.238M is starting concentration is 0.119M is i. (2) The average rate of disappearance of HI from t=0s to t=522s is Ms1. (3) The average rate of disappearance of HI from t=522s to t=783s is Ms1 (4) Based on these data, the rate constant for this order reaction is Ms1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


