Question: solve and show work Use the References to access important values if needed for this question. In a study of the decomposition of hydrogen iodide
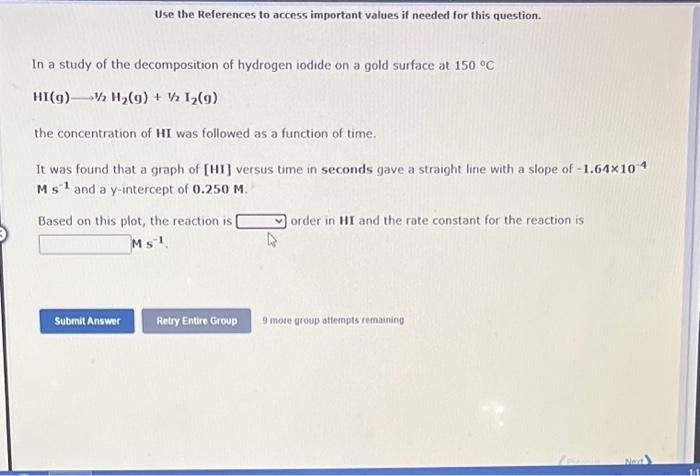
Use the References to access important values if needed for this question. In a study of the decomposition of hydrogen iodide on a gold surface at 150C HI(g)1/2H2(g)+1/2I2(g) the concentration of HI was followed as a function of time. It was found that a graph of [HI] versus time in seconds gave a straight line with a slope of 1.64104 Ms1 and a y-intercept of 0.250M. Based on this plot, the reaction is order in HI and the rate constant for the reaction is Ms1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


