Question: Shown below is a flowchart process in which acetic acid (CH,COOH) is extracted from a mixture of acetie acid and water into 1-hexanol (C,H30H),
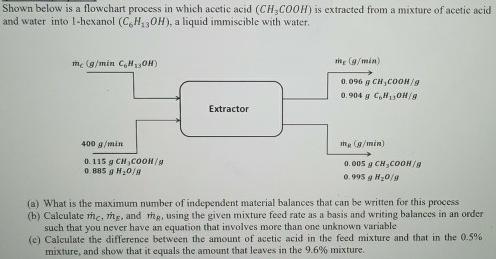
Shown below is a flowchart process in which acetic acid (CH,COOH) is extracted from a mixture of acetie acid and water into 1-hexanol (C,H30H), a liquid immiscible with water. me (g/min C,H13OH) 0.096 g CH,COOH/g 0.904 g C, OH/a Extractor 400 g/min ine Cg/min) 0. 115 g CH,CO0H/g O. 885 g H20/a 0.005 g CH,COON/ 0. 995 g H0/9 (a) What is the maximum number of independent material balances that can be written for this process (b) Calculate rme, mg, and thR, using the given mixture feed rate as a basis and writing balances in an order such that you never have an equation that involves more than one unknown variable (e) Calculate the difference between the amount of acetic acid in the feed mixture and that in the 0.5% mixture, and show that it equals the amount that leaves in the 9.6% mixture.
Step by Step Solution
There are 3 Steps involved in it
ME Mc Colminko HGOH 400 glmin gmin sobre g Cho Cookly 09049 Co the ... View full answer

Get step-by-step solutions from verified subject matter experts


