Question: Liquid extraction is an operation used to separate the components of a liquid mixture of two or more species. In the simplest case, the mixture
Liquid extraction is an operation used to separate the components of a liquid mixture of two or more species. In the simplest case, the mixture contains two components: a solute (A) and a Liquid solvent (B). The mixture is contacted in an agitated vessel with a second liquid solvent (C) that has two key properties: A dissolves in it. and B is immiscible or nearly immiscible with it, (For example. B may be water. C a hydrocarbon oil, and A a species that dissolves in both water and oil) Some of the A transfers from B to C, and then the B-rich phase (the raffinate) and the C-rich phase (the extract) from each other in A settling tank. If the raffinate is then contacted with fresh C in another stage, more A will be transferred from it. This process can be repeated until essentially all of the A has been extracted from the B. Shown below is a flowchart of a process in which acetic acid (A) is extracted from a mixture of acetic acid and water (B) into 1-hexanol (C), a liquid immiscible with water.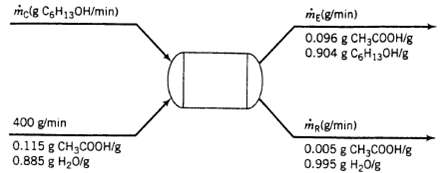
(a) What is the maximum number of independent material balances that can be written for this process?
(b) Calculate mC, mE and mR, using the given mixture feed rate as a basis and writing balances in an order such that you never have an equation that involves more than one unknown variable.
(c) Calculate the difference between the amount of acetic acid in the feed mixture and that in the 0.5% mixture, and show that it equals the amount that leaves in the 9.6% mixture.
(d) Acetic acid is relatively difficult to separate completely from water by distillation (see Problem 4.6) and relatively easy to separate from hexanol by distillation Sketch a flowchart of a two-unit process that might be used to recover nearly pure acetic acid from an acetic acid?water mixture.
mclg C6H130H/min) 400 g/min 0.115 g CHCOOH/g 0.885 g H0/g me(g/min) 0.096 g CH3COOH/g 0.904 g C6H130H/g me(g/min) 0.005 g CHCOOH/g 0.995 g H0lg
Step by Step Solution
3.55 Rating (155 Votes )
There are 3 Steps involved in it
a b C 3 independent balances one for each species 400 g 0885 g HO m g 0995 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (106).pdf
180 KBs PDF File
13-E-C-E-C-P (106).docx
120 KBs Word File


