Question: Sme data with 5 set questions Consider a water sample with the following ion concentrations. Assume that no particulate form exists in the water sample
Sme data with 5 set questions
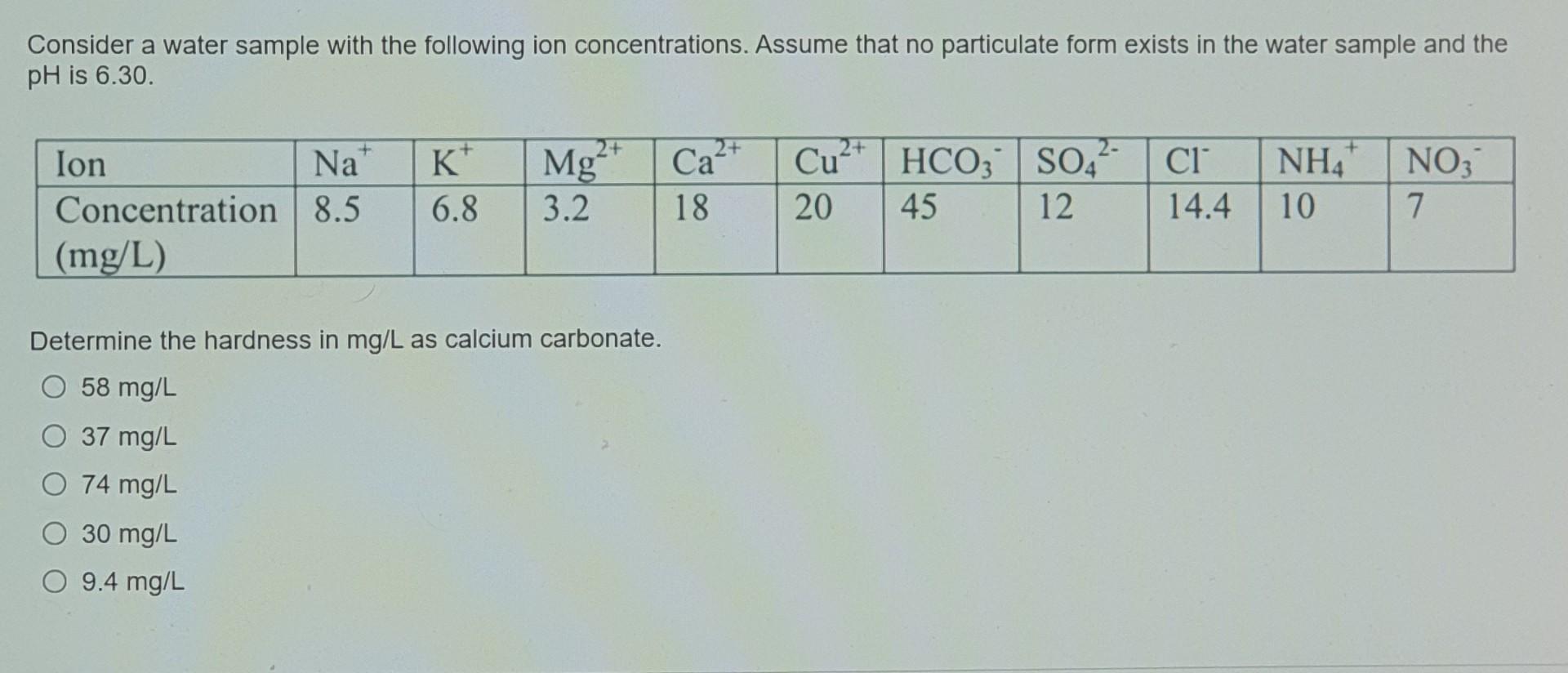




Consider a water sample with the following ion concentrations. Assume that no particulate form exists in the water sample and the pH is 6.30. + 2+ + lon Na Concentration 8.5 (mg/L) K 6.8 Mg 3.2 2+ Ca 18 2+ Cu HCO3 20 45 2- SO4 12 CI 14.4 NH4 10 NO3 7 Determine the hardness in mg/L as calcium carbonate. O 58 mg/L O 37 mg/L O 74 mg/L 30 mg/L O 9.4 mg/L Determine the alkalinity in mg/L as calcium carbonate. O 30 mg/L O 9.4 mg/L O 58 mg/L O 74 mg/L O 37 mg/L Ammonia is known to be toxic to fish when its concentration is greater than 0.5 mg/L of nitrogen. Under what pH condition will the water be toxic to fish? Keq of ammonia is 1.8 x 109. O 7.3 6.8 8.1 O 7.6 O 6.4 Determine the total nitrogen in mg/L. O 74 mg/L O 37 mg/L O 58 mg/L O 30 mg/L O 9.4 mg/L Copper will precipitate as copper hydroxide when the pH is increased. What is the pH that wil it start to precipitate? Ksp of copper hydroxide is 2 x 10-19 6.4 8.1 076 68 73
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


