Question: . . Solubility refers to the ability of an ionic compound to dissolve in water. More soluble means more dissolves. At the particle level, when

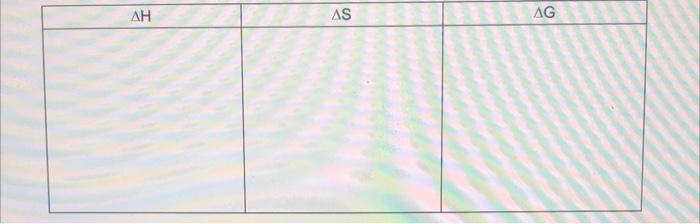

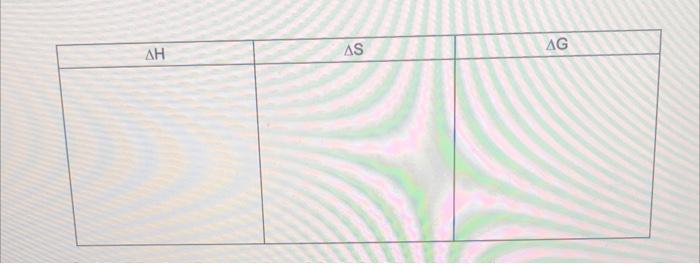
. . "Solubility" refers to the ability of an ionic compound to dissolve in water. More soluble means more dissolves. At the particle level, when an ionic solid (salt) "dissolves" in water, The ionic lattice of the "soluble salt" The ionic lattice There is solution formation since the salt ions Show the drawing below AS AG . At the particle level, when an ionic solid (salt) is "insoluble" in water, The ionic lattice of the "insoluble salt" remains intact The ionic lattice does not break up into the salt ions in solution. There is no solution formation since the salt ions do not become surrounded by water molecules in solution. Show the drawing below AG AS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


