Question: solution < Initial pH pH after H* addition pH after OH addition Buffer pH = 4.4 Buffer pH = 4.4 Buffer pH = 10.0
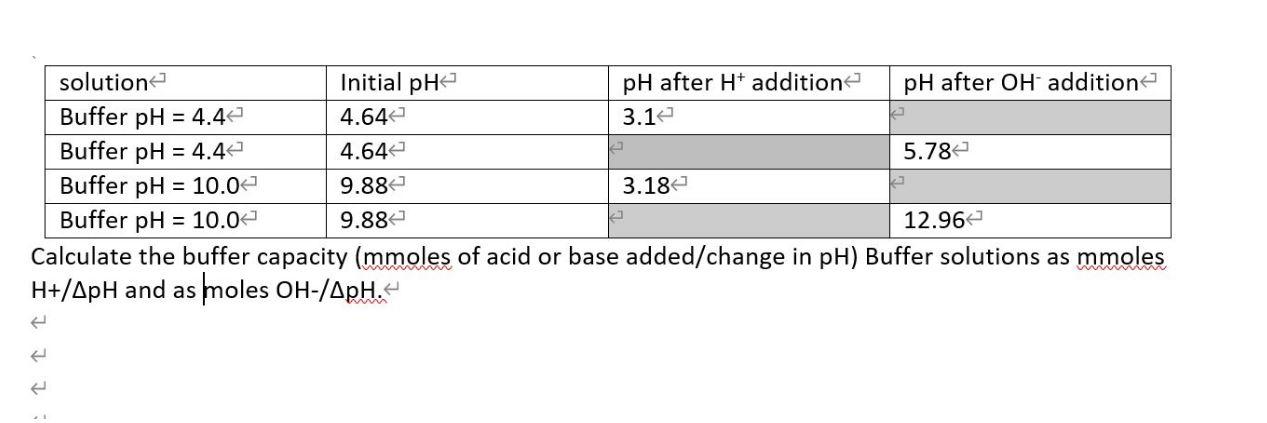
solution < Initial pH pH after H* addition pH after OH addition Buffer pH = 4.4 Buffer pH = 4.4 Buffer pH = 10.0 Buffer pH = 10.0 Calculate the buffer capacity (mmoles of acid or base added/change in pH) Buffer solutions as mmoles H+/ApH and as moles OH-/ApH. 4.64 3.1 %D 4.64 5.78e 9.88 3.18 9.88 12.96
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
To calculate the buffer capacity we need to find the amount of acid or base added and the change in ... View full answer

Get step-by-step solutions from verified subject matter experts


